Journal of Proteomics ( IF 2.8 ) Pub Date : 2020-10-09 , DOI: 10.1016/j.jprot.2020.104002 Jessica Molina-Franky 1 , David Fernando Plaza 2 , Carmen Merali 3 , Salim Merali 3 , Carlos Barrero 3 , Gabriela Arévalo-Pinzón 4 , Manuel Elkin Patarroyo 5 , Manuel Alfonso Patarroyo 6
|
Successful Plasmodium falciparum invasion of red blood cells includes the orderly execution of highly specific receptor-ligand molecular interactions between the parasite's proteins and the red blood cell membrane proteins. There is a growing need for elucidating receptor-ligand pairings, which will help in understanding the parasite's biology and provide the fundamental basis for developing prophylactic or therapeutic alternatives leading to mitigating or eliminating this type of malaria.
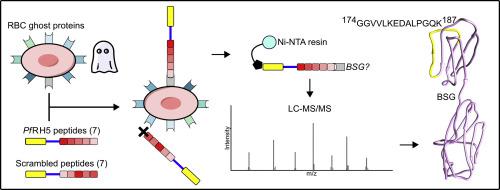
We have thus used Plasmodium falciparum RH5 - derived peptides and ghost red blood cell proteins in synthetic peptide affinity capture assays to identify important host receptors used by Plasmodium spp. in the invasion of red blood cells. LC-MS/MS analysis confirmed the extensively described interaction between PfRH5 and the basigin receptor on the red blood cell membrane.
As shown here, tagged synthetic peptides displaying high binding ability to erythrocytes can be used to identify receptors present in protein extracts from ghost red blood cells via affinity capture and LC-MS/MS.
Significance
The article describes a novel approach for identifying red blood cell receptors based on the ability of synthetic peptides having high red blood cell binding capacity to capture Plasmodium spp. receptors on proteins extracted from ghost red blood cells. Specifically, novel methods to identify Plasmodium falciparum reticulocyte binding protein homolog 5 PfRH5 and basigin interaction using a combination of affinity capture and LC-MS/MS assays is described. Identification of these host RBC receptors interacting with malarial parasite proteins is of utmost importance in studying the disease's pathogenesis and will provide crucial information in understanding the parasite's biology. In addition, data from these studies can be used to identify potential therapeutic target(s) to mitigate or eliminate this debilitating disease.
中文翻译:

肽介导的亲和捕获和 LC-MS/MS 鉴定参与疟原虫入侵的宿主受体的新平台
恶性疟原虫成功侵入红细胞包括寄生虫蛋白质和红细胞膜蛋白之间高度特异性受体-配体分子相互作用的有序执行。越来越需要阐明受体-配体配对,这将有助于了解寄生虫的生物学特性,并为开发可减轻或消除此类疟疾的预防性或治疗性替代方案奠定基础。
因此,我们在合成肽亲和捕获试验中使用了恶性疟原虫RH5 衍生肽和鬼红细胞蛋白,以鉴定疟原虫属使用的重要宿主受体。在红细胞的侵袭中。LC-MS/MS 分析证实了广泛描述的Pf RH5 与红细胞膜上的basigin 受体之间的相互作用。
如此处所示,通过亲和捕获和 LC-MS/MS,显示出与红细胞高结合能力的标记合成肽可用于识别鬼红细胞蛋白质提取物中存在的受体。
意义
该文章描述了一种基于具有高红细胞结合能力的合成肽捕获疟原虫属的能力来识别红细胞受体的新方法。从幽灵红细胞中提取的蛋白质上的受体。具体而言,鉴定恶性疟原虫网织红细胞结合蛋白同源物 5 Pf的新方法描述了结合使用亲和捕获和 LC-MS/MS 分析的 RH5 和 basigin 相互作用。鉴定与疟疾寄生虫蛋白相互作用的这些宿主红细胞受体对于研究疾病的发病机制至关重要,并将为了解寄生虫的生物学提供重要信息。此外,来自这些研究的数据可用于确定潜在的治疗目标,以减轻或消除这种使人衰弱的疾病。











































 京公网安备 11010802027423号
京公网安备 11010802027423号