Journal of Allergy and Clinical Immunology ( IF 11.4 ) Pub Date : 2020-10-09 , DOI: 10.1016/j.jaci.2020.09.033 Zuochen Du 1 , Anwei Chen 2 , Lu Huang 3 , Xin Dai 4 , Qiuyue Chen 4 , Di Yang 3 , Liling Li 3 , Heather Miller 5 , Lisa Westerberg 6 , Yuan Ding 7 , Xuemei Tang 8 , Masato Kubo 9 , Liping Jiang 3 , Xiaodong Zhao 3 , Hua Wang 10 , Chaohong Liu 4
|
Background
STAT3 or dedicator of cytokinesis protein 8 (Dock8) loss-of-function (LOF) mutations cause hyper-IgE syndrome. The role of abnormal T-cell function has been extensively investigated; however, the contribution of B-cell–intrinsic dysfunction to elevated IgE levels is unclear.
Objective
We sought to determine the underlying molecular mechanism of how STAT3 regulates B-cell receptor (BCR) signaling, B-cell differentiation, and IgE production.
Methods
We used samples from patients with STAT3 LOF mutation and samples from the STAT3 B-cell–specific knockout (KO) mice Mb1Cre Stat3flox/flox mice (B-STAT3 KO) to investigate the mechanism of hyper-IgE syndrome.
Results
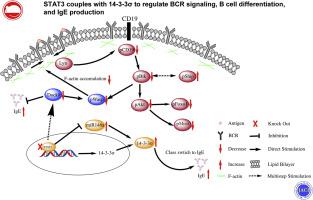
We found that the peripheral B-cell homeostasis in B-STAT3 KO mice mimicked the phenotype of patients with STAT3 LOF mutation, having decreased levels of follicular and germinal center B cells but increased levels of marginal zone and IgE+ B cells. Furthermore, B-STAT3 KO B cells had reduced BCR signaling following antigenic stimulation owing to reduced BCR clustering and decreased accumulation of Wiskott-Aldrich syndrome protein and F-actin. Excitingly, a central hub protein, 14-3-3σ, which is essential for the increase in IgE production, was enhanced in the B cells of B-STAT3 KO mice and patients with STAT3 LOF mutation. The increase of 14-3-3σ was associated with increased expression of the upstream mediator, microRNA146A. Inhibition of 14-3-3σ with R18 peptide in B-STAT3 KO mice rescued the BCR signaling, follicular, germinal center, and IgE+ B-cell differentiation to the degree seen in wild-type mice.
Conclusions
Altogether, our study has established a novel regulatory pathway of STAT3-miRNA146A-14-3-3σ to regulate BCR signaling, peripheral B-cell differentiation, and IgE production.
中文翻译:

STAT3 与 14-3-3σ 偶联来调节 BCR 信号传导、B 细胞分化和 IgE 产生
背景
STAT3 或胞质分裂蛋白 8 (Dock8) 功能丧失 (LOF) 突变会导致高 IgE 综合征。 T 细胞功能异常的作用已得到广泛研究;然而,B 细胞内在功能障碍对 IgE 水平升高的影响尚不清楚。
客观的
我们试图确定 STAT3 如何调节 B 细胞受体 (BCR) 信号传导、B 细胞分化和 IgE 产生的潜在分子机制。
方法
我们使用 STAT3 LOF 突变患者的样本和 STAT3 B 细胞特异性敲除 (KO) 小鼠Mb1 Cre Stat3 flox/flox小鼠 (B-STAT3 KO) 的样本来研究高 IgE 综合征的机制。
结果
我们发现 B-STAT3 KO 小鼠的外周 B 细胞稳态与 STAT3 LOF 突变患者的表型相似,滤泡和生发中心 B 细胞水平降低,但边缘区和 IgE + B 细胞水平升高。此外,B-STAT3 KO B 细胞在抗原刺激后减少了 BCR 信号传导,这是由于 BCR 聚集减少以及 Wiskott-Aldrich 综合征蛋白和 F-肌动蛋白积累减少。令人兴奋的是,一种对于增加 IgE 产生至关重要的中枢蛋白 14-3-3σ 在 B-STAT3 KO 小鼠和 STAT3 LOF 突变患者的 B 细胞中得到增强。 14-3-3σ 的增加与上游介质 microRNA146A 表达的增加相关。在 B-STAT3 KO 小鼠中用 R18 肽抑制 14-3-3σ 可挽救 BCR 信号传导、滤泡、生发中心和 IgE + B 细胞分化,达到野生型小鼠中所见的程度。
结论
总之,我们的研究建立了一条新的 STAT3-miRNA146A-14-3-3σ 调节途径来调节 BCR 信号传导、外周 B 细胞分化和 IgE 产生。











































 京公网安备 11010802027423号
京公网安备 11010802027423号