当前位置:
X-MOL 学术
›
Hydrometallurgy
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective leaching of vanadium from vanadium-chromium slag using sodium bicarbonate solution and subsequent in-situ preparation of flower-like VS2
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.hydromet.2020.105498 Jing Wen , Tao Jiang , Shapkat Arken
Hydrometallurgy ( IF 4.8 ) Pub Date : 2020-12-01 , DOI: 10.1016/j.hydromet.2020.105498 Jing Wen , Tao Jiang , Shapkat Arken
|
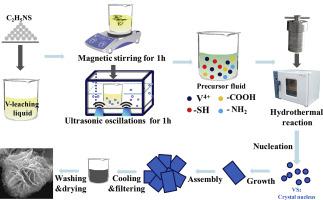
Abstract A novel leaching method for selectively separating vanadium from calcification roasted vanadium‑chromium slag (V Cr slag) was proposed, using sodium bicarbonate (NaHCO3) as the leaching medium. Vanadium disulfide (VS2), which can be used as an energy storage material, was prepared in-situ by a hydrothermal reaction from the vanadium-containing leaching solution, without purification. VS2 was characterized by XRD, SEM, XPS, and BET. Results show that the leaching extent of vanadium was 92.98% when the calcification roasted materials were treated with 80 g/L NaHCO3 solution and leached at 100 °C for 120 min. Additionally, only 1.84% silicon, 0.05% chromium, 0.06% iron, and 0.05% manganese were co-leached along vanadium, meaning that an effective selective vanadium extraction was achieved. The leaching solution was treated with thioacetamide (TAA) in a hydrothermal reaction at 140 °C for 16 h, which resulted in a vanadium recovery of 96.00%. The reaction generated flower-like VS2, with vanadium and sulfur in the form of V4+ and S2−. The BET specific surface area of the flower-like VS2 was 21.63 m2/g. As an anode material for Li-ion batteries, the initial discharge capacity of the flower-like VS2 reached 1119.83 mAh/g at current density of 100 mA/g, which was favorable for ion storage. This process not only achieved the selective extraction of vanadium, but also provided a new mechanism to shorten the preparation process of VS2 while generating a product with added value.
中文翻译:

使用碳酸氢钠溶液从钒铬渣中选择性浸出钒并随后原位制备花状 VS2
摘要 以碳酸氢钠(NaHCO3)为浸出介质,提出了一种从钙化焙烧钒铬渣(V Cr 渣)中选择性分离钒的浸出方法。可用作储能材料的二硫化钒 (VS2) 是通过水热反应从含钒浸出液中原位制备的,无需纯化。VS2 通过 XRD、SEM、XPS 和 BET 进行表征。结果表明,钙化焙烧物料用80 g/L NaHCO3溶液在100℃浸出120 min时,钒的浸出率为92.98%。此外,只有 1.84% 的硅、0.05% 的铬、0.06% 的铁和 0.05% 的锰沿钒共同浸出,这意味着实现了有效的选择性钒提取。浸出液用硫代乙酰胺 (TAA) 在 140°C 的水热反应中处理 16 小时,钒的回收率为 96.00%。该反应生成花状 VS2,钒和硫以 V4+ 和 S2- 的形式存在。花状VS2的BET比表面积为21.63 m2/g。作为锂离子电池的负极材料,花状VS2在100 mA/g的电流密度下的初始放电容量达到1119.83 mAh/g,有利于离子存储。该工艺不仅实现了钒的选择性提取,而且为缩短VS2的制备过程同时产生具有附加值的产品提供了一种新的机制。钒和硫以 V4+ 和 S2- 的形式存在。花状VS2的BET比表面积为21.63 m2/g。作为锂离子电池的负极材料,花状VS2在100 mA/g的电流密度下的初始放电容量达到1119.83 mAh/g,有利于离子存储。该工艺不仅实现了钒的选择性提取,而且为缩短VS2的制备过程同时产生具有附加值的产品提供了一种新的机制。钒和硫以 V4+ 和 S2- 的形式存在。花状VS2的BET比表面积为21.63 m2/g。作为锂离子电池的负极材料,花状VS2在100 mA/g的电流密度下的初始放电容量达到1119.83 mAh/g,有利于离子存储。该工艺不仅实现了钒的选择性提取,而且为缩短VS2的制备过程同时产生具有附加值的产品提供了一种新的机制。
更新日期:2020-12-01
中文翻译:

使用碳酸氢钠溶液从钒铬渣中选择性浸出钒并随后原位制备花状 VS2
摘要 以碳酸氢钠(NaHCO3)为浸出介质,提出了一种从钙化焙烧钒铬渣(V Cr 渣)中选择性分离钒的浸出方法。可用作储能材料的二硫化钒 (VS2) 是通过水热反应从含钒浸出液中原位制备的,无需纯化。VS2 通过 XRD、SEM、XPS 和 BET 进行表征。结果表明,钙化焙烧物料用80 g/L NaHCO3溶液在100℃浸出120 min时,钒的浸出率为92.98%。此外,只有 1.84% 的硅、0.05% 的铬、0.06% 的铁和 0.05% 的锰沿钒共同浸出,这意味着实现了有效的选择性钒提取。浸出液用硫代乙酰胺 (TAA) 在 140°C 的水热反应中处理 16 小时,钒的回收率为 96.00%。该反应生成花状 VS2,钒和硫以 V4+ 和 S2- 的形式存在。花状VS2的BET比表面积为21.63 m2/g。作为锂离子电池的负极材料,花状VS2在100 mA/g的电流密度下的初始放电容量达到1119.83 mAh/g,有利于离子存储。该工艺不仅实现了钒的选择性提取,而且为缩短VS2的制备过程同时产生具有附加值的产品提供了一种新的机制。钒和硫以 V4+ 和 S2- 的形式存在。花状VS2的BET比表面积为21.63 m2/g。作为锂离子电池的负极材料,花状VS2在100 mA/g的电流密度下的初始放电容量达到1119.83 mAh/g,有利于离子存储。该工艺不仅实现了钒的选择性提取,而且为缩短VS2的制备过程同时产生具有附加值的产品提供了一种新的机制。钒和硫以 V4+ 和 S2- 的形式存在。花状VS2的BET比表面积为21.63 m2/g。作为锂离子电池的负极材料,花状VS2在100 mA/g的电流密度下的初始放电容量达到1119.83 mAh/g,有利于离子存储。该工艺不仅实现了钒的选择性提取,而且为缩短VS2的制备过程同时产生具有附加值的产品提供了一种新的机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号