Biochimica et Biophysica Acta (BBA) - Bioenergetics ( IF 3.4 ) Pub Date : 2020-10-09 , DOI: 10.1016/j.bbabio.2020.148324 Gregory S. Orf , Kevin E. Redding
|
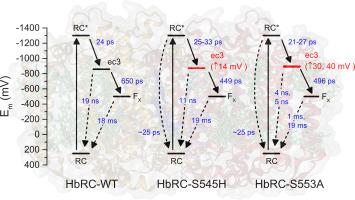
All known Type I photochemical reaction center protein complexes contain a form of the pigment chlorophyll a in their primary electron acceptor site (termed ec3). In the reaction center from the primitive heliobacteria (HbRC), all of the pigment cofactors are bacteriochlorophyll g except in the ec3 sites, which contain 81-hydroxychlorophyll a. To explore the energetic flexibility of this site, we performed site-directed mutagenesis on two of the amino acids of the PshA core polypeptide responsible for coordinating the 81-hydroxychlorophyll a. These two amino acids are serine-545, which coordinates the central Mg(II) through an intermediary water molecule, and serine-553, which participates in a hydrogen bond with the 131-keto O atom. Mutagenesis of serine-545 to histidine (S545H) changes how the chlorophyll's central Mg(II) is coordinated, with the result of decreasing the chlorophyll's site energy. Mutagenesis of serine-545 to methionine (S545M), which was made to mimic the ec3 site of Photosystem I, abolishes chlorophyll binding and charge separation altogether. Mutagenesis of serine-553 to alanine (S553A) removes the aforementioned hydrogen bond, increasing the site energy of the chlorophyll. In the S545H and S553A mutants, the forward and reverse electron transfer rates from ec3 are both faster. This coincides with a decrease in both the quantum yield of initial charge separation and the overall photochemical quantum yield. Taken together, these data indicate that wild-type HbRC is optimized for overall photochemical efficiency, rather than just for maximizing the forward electron transfer rate. The necessity for a chlorophyll a derivative at the ec3 site is also discussed.
中文翻译:

通过协调氨基酸取代来扰乱嗜氧菌反应中心主要受体叶绿素位点
所有已知的I型光化学反应中心蛋白复合物在其主要电子受体位点(称为ec3)中都含有一种形式的色素叶绿素a。在原始Heliobacteria(HbRC)的反应中心,所有色素辅因子都是细菌叶绿素g,除了在ec3位点中含有8个1-羟基叶绿素a。为了探索该位点的能量灵活性,我们对负责协调8 1-羟基叶绿素a的PshA核心多肽的两个氨基酸进行了定点诱变。这两个氨基酸是丝氨酸545和丝氨酸553,它们通过中间水分子与中心Mg(II)结合,丝氨酸553与13 1-酮基O原子。丝氨酸545突变为组氨酸(S545H)改变了叶绿素中央Mg(II)的配位方式,从而降低了叶绿素的位能。丝氨酸545突变为蛋氨酸(S545M)的目的是模仿光系统I的ec3位点,从而消除了叶绿素结合和电荷分离。丝氨酸553向丙氨酸的诱变(S553A)除去了上述氢键,从而增加了叶绿素的位能。在S545H和S553A突变体中,来自ec3的正向和反向电子转移速率都更快。这与初始电荷分离的量子产率和整个光化学量子产率的降低都相符。综上所述,这些数据表明,野生型HbRC针对整体光化学效率进行了优化,而不仅仅是为了最大化正向电子传输速率。叶绿素的必要性还讨论了ec3网站上的衍生物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号