当前位置:
X-MOL 学术
›
Eur. J. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Prophylactic and therapeutic HBV vaccination by an HBs‐expressing cytomegalovirus vector lacking an interferon antagonist in mice
European Journal of Immunology ( IF 4.5 ) Pub Date : 2020-10-07 , DOI: 10.1002/eji.202048780 Hongming Huang 1 , Meike Rückborn 2 , Vu Thuy Khanh Le‐Trilling 2 , Dan Zhu 1 , Shangqing Yang 1 , Wenqing Zhou 1 , Xuecheng Yang 1 , Xuemei Feng 1 , Yinping Lu 1 , Mengji Lu 2 , Ulf Dittmer 2 , Dongliang Yang 1 , Mirko Trilling 2 , Jia Liu 1
European Journal of Immunology ( IF 4.5 ) Pub Date : 2020-10-07 , DOI: 10.1002/eji.202048780 Hongming Huang 1 , Meike Rückborn 2 , Vu Thuy Khanh Le‐Trilling 2 , Dan Zhu 1 , Shangqing Yang 1 , Wenqing Zhou 1 , Xuecheng Yang 1 , Xuemei Feng 1 , Yinping Lu 1 , Mengji Lu 2 , Ulf Dittmer 2 , Dongliang Yang 1 , Mirko Trilling 2 , Jia Liu 1
Affiliation
|
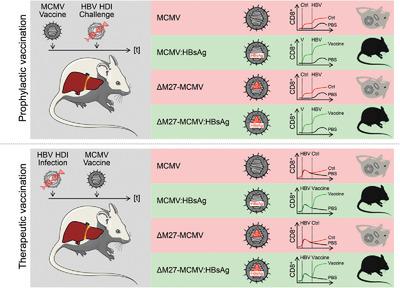
Cytomegalovirus (CMV)‐based vaccines show promising effects against chronic infections in nonhuman primates. Therefore, we examined the potential of hepatitis B virus (HBV) vaccines based on mouse CMV (MCMV) vectors expressing the small HBsAg. Immunological consequences of vaccine virus attenuation were addressed by either replacing the dispensable gene m157 (“MCMV‐HBsȍ) or the gene M27 (“ΔM27‐HBs”), the latter encodes a potent IFN antagonist targeting the transcription factor STAT2. M27 was chosen, since human CMV encodes an analogous gene product, which also induced proteasomal STAT2 degradation by exploiting Cullin RING ubiquitin ligases. Vaccinated mice were challenged with HBV through hydrodynamic injection. MCMV‐HBs and ΔM27‐HBs vaccination achieved accelerated HBV clearance in serum and liver as well as robust HBV‐specific CD8+ T‐cell responses. When we explored the therapeutic potential of MCMV‐based vaccines, especially the combination of ΔM27‐HBs prime and DNA boost vaccination resulted in increased intrahepatic HBs‐specific CD8+ T‐cell responses and HBV clearance in persistently infected mice. Our results demonstrated that vaccines based on a replication competent MCMV attenuated through the deletion of an IFN antagonist targeting STAT2 elicit robust anti‐HBV immune responses and mediate HBV clearance in mice in prophylactic and therapeutic immunization regimes.
中文翻译:

缺乏干扰素拮抗剂的表达HBs的巨细胞病毒载体对小鼠的预防性和治疗性HBV疫苗接种
基于巨细胞病毒(CMV)的疫苗对非人类灵长类动物的慢性感染显示出令人鼓舞的效果。因此,我们检查了基于表达小HBsAg的小鼠CMV(MCMV)载体的乙型肝炎病毒(HBV)疫苗的潜力。疫苗病毒减毒的免疫学后果可通过替换可分配的基因m157(“MCMV-HBsȍ”)或基因M27(“ΔM27-HBs”)来解决,后者编码一种有效的针对转录因子STAT2的IFN拮抗剂。M27由于人CMV编码类似的基因产物,因此也被选中,因为该产物还通过利用Cullin RING泛素连接酶诱导了蛋白酶体STAT2降解。通过流体动力注射用HBV攻击疫苗接种的小鼠。接种MCMV-HBs和ΔM27-HBs可以加速血清和肝脏中的HBV清除,并具有强大的HBV特异性CD8 + T细胞反应。当我们探索基于MCMV的疫苗的治疗潜力时,尤其是结合使用ΔM27-HBs初免和DNA加强疫苗接种后,肝内HBs特异性CD8 +增加持续感染小鼠的T细胞反应和HBV清除率。我们的结果表明,基于复制能力MCMV的疫苗可通过删除靶向STAT2的IFN拮抗剂而减弱,从而在预防性和治疗性免疫方案中引起强大的抗HBV免疫反应并介导HBV清除。
更新日期:2020-10-07
中文翻译:

缺乏干扰素拮抗剂的表达HBs的巨细胞病毒载体对小鼠的预防性和治疗性HBV疫苗接种
基于巨细胞病毒(CMV)的疫苗对非人类灵长类动物的慢性感染显示出令人鼓舞的效果。因此,我们检查了基于表达小HBsAg的小鼠CMV(MCMV)载体的乙型肝炎病毒(HBV)疫苗的潜力。疫苗病毒减毒的免疫学后果可通过替换可分配的基因m157(“MCMV-HBsȍ”)或基因M27(“ΔM27-HBs”)来解决,后者编码一种有效的针对转录因子STAT2的IFN拮抗剂。M27由于人CMV编码类似的基因产物,因此也被选中,因为该产物还通过利用Cullin RING泛素连接酶诱导了蛋白酶体STAT2降解。通过流体动力注射用HBV攻击疫苗接种的小鼠。接种MCMV-HBs和ΔM27-HBs可以加速血清和肝脏中的HBV清除,并具有强大的HBV特异性CD8 + T细胞反应。当我们探索基于MCMV的疫苗的治疗潜力时,尤其是结合使用ΔM27-HBs初免和DNA加强疫苗接种后,肝内HBs特异性CD8 +增加持续感染小鼠的T细胞反应和HBV清除率。我们的结果表明,基于复制能力MCMV的疫苗可通过删除靶向STAT2的IFN拮抗剂而减弱,从而在预防性和治疗性免疫方案中引起强大的抗HBV免疫反应并介导HBV清除。










































 京公网安备 11010802027423号
京公网安备 11010802027423号