当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A solution‐free crystal‐mounting platform for native SAD
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-10-08 , DOI: 10.1107/s2059798320011584 Jian Yu 1 , Akira Shinoda 1 , Koji Kato 1 , Isao Tanaka 1 , Min Yao 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-10-08 , DOI: 10.1107/s2059798320011584 Jian Yu 1 , Akira Shinoda 1 , Koji Kato 1 , Isao Tanaka 1 , Min Yao 1
Affiliation
|
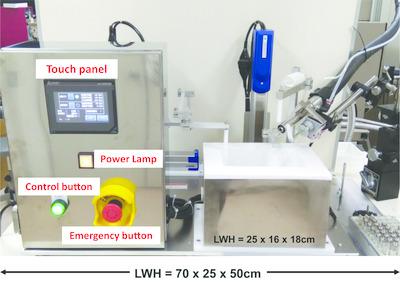
The native SAD phasing method uses the anomalous scattering signals from the S atoms contained in most proteins, the P atoms in nucleic acids or other light atoms derived from the solution used for crystallization. These signals are very weak and careful data collection is required, which makes this method very difficult. One way to enhance the anomalous signal is to use long‐wavelength X‐rays; however, these wavelengths are more strongly absorbed by the materials in the pathway. Therefore, a crystal‐mounting platform for native SAD data collection that removes solution around the crystals has been developed. This platform includes a novel solution‐free mounting tool and an automatic robot, which extracts the surrounding solution, flash‐cools the crystal and inserts the loop into a UniPuck cassette for use in the synchrotron. Eight protein structures (including two new structures) have been successfully solved by the native SAD method from crystals prepared using this platform.
中文翻译:

适用于本机SAD的无解决方案的晶体安装平台
天然的SAD相控方法使用来自大多数蛋白质中包含的S原子,核酸中的P原子或其他用于结晶的溶液的轻原子的异常散射信号。这些信号非常微弱,需要仔细收集数据,这使此方法非常困难。增强异常信号的一种方法是使用长波长X射线。然而,这些波长被路径中的材料更强烈地吸收。因此,已经开发了用于本地SAD数据收集的晶体安装平台,该平台可去除晶体周围的溶液。该平台包括一个无需解决方案的新型安装工具和一个自动机器人,该机器人可以提取周围的溶液,对晶体进行快速冷却,然后将环路插入UniPuck盒中,以用于同步加速器。
更新日期:2020-10-08
中文翻译:

适用于本机SAD的无解决方案的晶体安装平台
天然的SAD相控方法使用来自大多数蛋白质中包含的S原子,核酸中的P原子或其他用于结晶的溶液的轻原子的异常散射信号。这些信号非常微弱,需要仔细收集数据,这使此方法非常困难。增强异常信号的一种方法是使用长波长X射线。然而,这些波长被路径中的材料更强烈地吸收。因此,已经开发了用于本地SAD数据收集的晶体安装平台,该平台可去除晶体周围的溶液。该平台包括一个无需解决方案的新型安装工具和一个自动机器人,该机器人可以提取周围的溶液,对晶体进行快速冷却,然后将环路插入UniPuck盒中,以用于同步加速器。









































 京公网安备 11010802027423号
京公网安备 11010802027423号