当前位置:
X-MOL 学术
›
Acta Cryst. D
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Plasticity, ligand conformation and enzyme action of Mycobacterium smegmatis MutT1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-10-08 , DOI: 10.1107/s2059798320010992 Prateek Raj 1 , S Karthik 1 , S M Arif 1 , U Varshney 2 , M Vijayan 1
Acta Crystallographica Section D ( IF 2.6 ) Pub Date : 2020-10-08 , DOI: 10.1107/s2059798320010992 Prateek Raj 1 , S Karthik 1 , S M Arif 1 , U Varshney 2 , M Vijayan 1
Affiliation
|
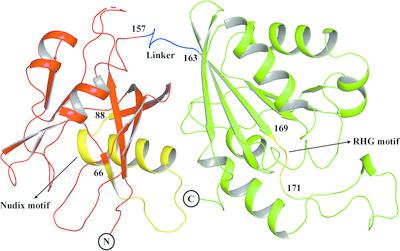
Mycobacterium smegmatis MutT1 (MsMutT1) is a sanitation enzyme made up of an N‐terminal Nudix hydrolase domain and a C‐terminal domain resembling a histidine phosphatase. It has been established that the action of MutT1 on 8‐oxo‐dGTP, 8‐oxo‐GTP and diadenosine polyphosphates is modulated by intermolecular interactions. In order to further explore this and to elucidate the structural basis of its differential action on 8‐oxo‐NTPs and unsubstituted NTPs, the crystal structures of complexes of MsMutT1 with 8‐oxo‐dGTP, GMPPNP and GMPPCP have been determined. Replacement soaking was used in order to ensure that the complexes were isomorphous to one another. Analysis of the structural data led to the elucidation of a relationship between the arrangements of molecules observed in the crystals, molecular plasticity and the action of the enzyme on nucleotides. The dominant mode of arrangement involving a head‐to‐tail sequence predominantly leads to the generation of NDPs. The other mode of packing arrangement appears to preferentially generate NMPs. This work also provides interesting insights into the dependence of enzyme action on the conformation of the ligand. The possibility of modulating the enzyme action through differences in intermolecular interactions and ligand conformations makes MsMutT1 a versatile enzyme.
中文翻译:

耻垢分枝杆菌 MutT1 的可塑性、配体构象和酶作用
耻垢分枝杆菌MutT1 ( Ms MutT1) 是一种卫生酶,由 N 端 Nudix 水解酶结构域和类似于组氨酸磷酸酶的 C 端结构域组成。已经确定 MutT1 对 8-oxo-dGTP、8-oxo-GTP 和二腺苷多磷酸的作用是通过分子间相互作用调节的。为了进一步探索这一点并阐明其对 8-oxo-NTP 和未取代的 NTP 的差异作用的结构基础,确定了Ms MutT1 与 8-oxo-dGTP、GMPPNP 和 GMPPCP 复合物的晶体结构。使用置换浸泡是为了确保复合物彼此同晶。对结构数据的分析阐明了晶体中观察到的分子排列、分子可塑性和酶对核苷酸的作用之间的关系。涉及头尾序列的主导排列模式主要导致 NDP 的生成。另一种堆积排列方式似乎优先生成 NMP。这项工作还为酶作用对配体构象的依赖性提供了有趣的见解。通过分子间相互作用和配体构象的差异调节酶作用的可能性使得MutT1成为一种多功能酶。
更新日期:2020-10-08
中文翻译:

耻垢分枝杆菌 MutT1 的可塑性、配体构象和酶作用
耻垢分枝杆菌MutT1 ( Ms MutT1) 是一种卫生酶,由 N 端 Nudix 水解酶结构域和类似于组氨酸磷酸酶的 C 端结构域组成。已经确定 MutT1 对 8-oxo-dGTP、8-oxo-GTP 和二腺苷多磷酸的作用是通过分子间相互作用调节的。为了进一步探索这一点并阐明其对 8-oxo-NTP 和未取代的 NTP 的差异作用的结构基础,确定了Ms MutT1 与 8-oxo-dGTP、GMPPNP 和 GMPPCP 复合物的晶体结构。使用置换浸泡是为了确保复合物彼此同晶。对结构数据的分析阐明了晶体中观察到的分子排列、分子可塑性和酶对核苷酸的作用之间的关系。涉及头尾序列的主导排列模式主要导致 NDP 的生成。另一种堆积排列方式似乎优先生成 NMP。这项工作还为酶作用对配体构象的依赖性提供了有趣的见解。通过分子间相互作用和配体构象的差异调节酶作用的可能性使得MutT1成为一种多功能酶。











































 京公网安备 11010802027423号
京公网安备 11010802027423号