当前位置:
X-MOL 学术
›
Eur. J. Nerosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
FKBP5 polymorphisms induce differential glucocorticoid responsiveness in primary CNS cells – First insights from novel humanized mice
European Journal of Neroscience ( IF 3.4 ) Pub Date : 2020-10-08 , DOI: 10.1111/ejn.14999 Verena Nold 1, 2 , Nadine Richter 1 , Bastian Hengerer 1 , Iris-Tatjana Kolassa 2 , Kelly Ann Allers 1
European Journal of Neroscience ( IF 3.4 ) Pub Date : 2020-10-08 , DOI: 10.1111/ejn.14999 Verena Nold 1, 2 , Nadine Richter 1 , Bastian Hengerer 1 , Iris-Tatjana Kolassa 2 , Kelly Ann Allers 1
Affiliation
|
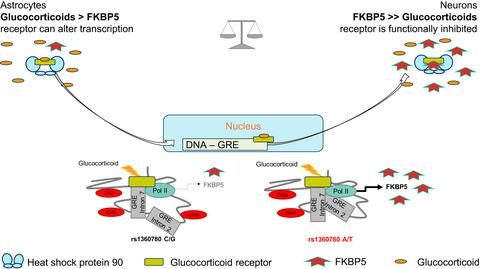
The brain is a central hub for integration of internal and external conditions and, thus, a regulator of the stress response. Glucocorticoids are the essential communicators of this response. Aberrations in glucocorticoid signaling are a common symptom in patients with psychiatric disorders. The gene FKBP5 encodes a chaperone protein that functionally inhibits glucocorticoid signaling and, thus, contributes to the regulation of stress. In the context of childhood trauma, differential expression of FKBP5 has been found in psychiatric patients compared to controls. These variations in expression levels of FKBP5 were reported to be associated with differences in stress responsiveness in human carriers of the single nucleotide polymorphism (SNP) rs1360780. Understanding the mechanisms underlying FKBP5 polymorphism‐associated glucocorticoid responsiveness in the CNS will lead to a better understanding of stress regulation or associated pathology. To study these mechanisms, two novel humanized mouse lines were generated. The lines carried either the risk (A/T) allele or the resilient (C/G) allele of rs1360780. Primary cells from CNS (astrocytes, microglia, and neurons) were analyzed for their basal expression levels of FKBP5 and their responsiveness to glucocorticoids. Differential expression of FKBP5 was found for these cell types and negatively correlated with the cellular glucocorticoid responsiveness. Astrocytes revealed the strongest transcriptional response, followed by microglia and neurons. Furthermore, the risk allele (A/T) was associated with greater induction of FKBP5 than the resilience allele. Novel FKBP5‐humanized mice display differential glucocorticoid responsiveness due to a single intronic SNP. The vulnerability to stress signaling in the shape of glucocorticoids in the brain correlated with FKBP5 expression levels. The strong responsiveness of astrocytes to glucocorticoids implies astrocytes play a prominent role in the stress response, and in FKBP5‐related differences in glucocorticoid signaling. The novel humanized mouse lines will allow for further study of the role that FKBP5 SNPs have in risk and resilience to stress pathology.
中文翻译:

FKBP5 多态性在原代 CNS 细胞中诱导不同的糖皮质激素反应——来自新型人源化小鼠的初步见解
大脑是整合内部和外部条件的中心枢纽,因此是压力反应的调节器。糖皮质激素是这种反应的重要传播者。糖皮质激素信号异常是精神疾病患者的常见症状。FKBP5基因编码一种伴侣蛋白,该蛋白在功能上抑制糖皮质激素信号传导,因此有助于调节压力。在儿童创伤的背景下,与对照组相比,在精神病患者中发现了FKBP5的差异表达。FKBP5表达水平的这些变化据报道,与单核苷酸多态性(SNP)rs1360780的人类携带者的应激反应差异有关。了解中枢神经系统中FKBP5多态性相关糖皮质激素反应的潜在机制将有助于更好地了解压力调节或相关病理学。为了研究这些机制,产生了两个新的人源化小鼠系。这些品系携带 rs1360780 的风险 (A/T) 等位基因或弹性 (C/G) 等位基因。分析来自 CNS(星形胶质细胞、小胶质细胞和神经元)的原代细胞的FKBP5基础表达水平及其对糖皮质激素的反应。FKBP5的差异表达发现这些细胞类型与细胞糖皮质激素反应呈负相关。星形胶质细胞显示最强的转录反应,其次是小胶质细胞和神经元。此外,与弹性等位基因相比,风险等位基因 (A/T) 与更大的FKBP5诱导相关。由于单个内含子 SNP,新型FKBP5人源化小鼠表现出不同的糖皮质激素反应性。大脑中糖皮质激素形式的压力信号易感性与FKBP5表达水平相关。星形胶质细胞对糖皮质激素的强烈反应意味着星形胶质细胞在应激反应和FKBP5中起重要作用糖皮质激素信号传导的相关差异。新的人源化小鼠系将允许进一步研究FKBP5 SNP 在压力病理学风险和恢复中的作用。
更新日期:2020-10-08
中文翻译:

FKBP5 多态性在原代 CNS 细胞中诱导不同的糖皮质激素反应——来自新型人源化小鼠的初步见解
大脑是整合内部和外部条件的中心枢纽,因此是压力反应的调节器。糖皮质激素是这种反应的重要传播者。糖皮质激素信号异常是精神疾病患者的常见症状。FKBP5基因编码一种伴侣蛋白,该蛋白在功能上抑制糖皮质激素信号传导,因此有助于调节压力。在儿童创伤的背景下,与对照组相比,在精神病患者中发现了FKBP5的差异表达。FKBP5表达水平的这些变化据报道,与单核苷酸多态性(SNP)rs1360780的人类携带者的应激反应差异有关。了解中枢神经系统中FKBP5多态性相关糖皮质激素反应的潜在机制将有助于更好地了解压力调节或相关病理学。为了研究这些机制,产生了两个新的人源化小鼠系。这些品系携带 rs1360780 的风险 (A/T) 等位基因或弹性 (C/G) 等位基因。分析来自 CNS(星形胶质细胞、小胶质细胞和神经元)的原代细胞的FKBP5基础表达水平及其对糖皮质激素的反应。FKBP5的差异表达发现这些细胞类型与细胞糖皮质激素反应呈负相关。星形胶质细胞显示最强的转录反应,其次是小胶质细胞和神经元。此外,与弹性等位基因相比,风险等位基因 (A/T) 与更大的FKBP5诱导相关。由于单个内含子 SNP,新型FKBP5人源化小鼠表现出不同的糖皮质激素反应性。大脑中糖皮质激素形式的压力信号易感性与FKBP5表达水平相关。星形胶质细胞对糖皮质激素的强烈反应意味着星形胶质细胞在应激反应和FKBP5中起重要作用糖皮质激素信号传导的相关差异。新的人源化小鼠系将允许进一步研究FKBP5 SNP 在压力病理学风险和恢复中的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号