当前位置:
X-MOL 学术
›
Electroanalysis
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu2O/CuO Electrocatalyst for Electrochemical Reduction of Carbon Dioxide to Methanol
Electroanalysis ( IF 2.7 ) Pub Date : 2020-10-07 , DOI: 10.1002/elan.202060265 Animesh Roy 1 , Harsharaj Jadhav 1 , Jeong Gil Seo 2
Electroanalysis ( IF 2.7 ) Pub Date : 2020-10-07 , DOI: 10.1002/elan.202060265 Animesh Roy 1 , Harsharaj Jadhav 1 , Jeong Gil Seo 2
Affiliation
|
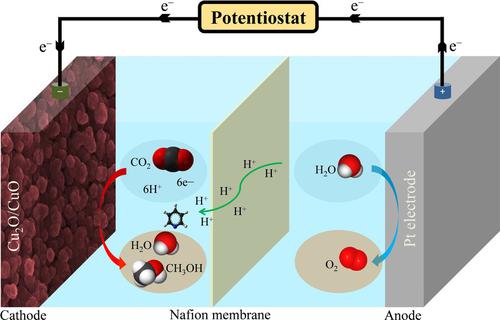
Herein, we report the controlled and direct fabrication of Cu2O/CuO thin film on the conductive nickel foam using electrodeposition route for the electrochemical reduction of carbon dioxide (CO2) to methanol. The electrocatalytic reduction was performed in CO2 saturated aqueous solution consisting of KHCO3, pyridine and HCl at room temperature. CO2 reduction was carried out at a constant potential of −1.3 V for 120 min to study the electrochemical performance of the prepared electrocatalysts. Cu2O/CuO shows better electrocatalytic activity with highest current density of 46 mA/cm2. The prepared catalyst can be an efficient and selective electrode for the production of methanol.
中文翻译:

用于将二氧化碳电化学还原为甲醇的Cu2O / CuO电催化剂
本文中,我们报道了使用电沉积途径将二氧化碳(CO 2)电化学还原为甲醇的方法,在导电镍泡沫上控制和直接制备Cu 2 O / CuO薄膜。在室温下,在由KHCO 3,吡啶和HCl组成的CO 2饱和水溶液中进行电催化还原。在-1.3 V的恒定电势下进行120分钟的CO 2还原,以研究制得的电催化剂的电化学性能。Cu 2 O / CuO具有更好的电催化活性,最大电流密度为46 mA / cm 2。所制备的催化剂可以是用于生产甲醇的有效且选择性的电极。
更新日期:2020-10-07
中文翻译:

用于将二氧化碳电化学还原为甲醇的Cu2O / CuO电催化剂
本文中,我们报道了使用电沉积途径将二氧化碳(CO 2)电化学还原为甲醇的方法,在导电镍泡沫上控制和直接制备Cu 2 O / CuO薄膜。在室温下,在由KHCO 3,吡啶和HCl组成的CO 2饱和水溶液中进行电催化还原。在-1.3 V的恒定电势下进行120分钟的CO 2还原,以研究制得的电催化剂的电化学性能。Cu 2 O / CuO具有更好的电催化活性,最大电流密度为46 mA / cm 2。所制备的催化剂可以是用于生产甲醇的有效且选择性的电极。











































 京公网安备 11010802027423号
京公网安备 11010802027423号