Journal of Molecular and Cellular Cardiology ( IF 4.9 ) Pub Date : 2020-10-08 , DOI: 10.1016/j.yjmcc.2020.09.015 Amir Aghajanian 1 , Hua Zhang 1 , Brian K Buckley 1 , Erika S Wittchen 1 , Willa Y Ma 1 , James E Faber 1
|
Rationale
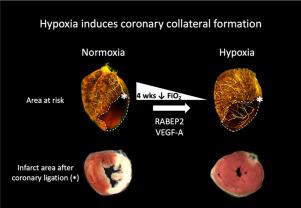
Collateral vessels lessen myocardial ischemia when acute or chronic coronary obstruction occurs. It has long been assumed that although native (pre-existing) collaterals enlarge in obstructive disease, new collaterals do not form in the adult. However, the latter was recently shown to occur after coronary artery ligation. Understanding the signals that drive this process is challenged by the difficulty in studying collateral vessels directly and the complex milieu of signaling pathways, including cell death, induced by ligation. Herein we show that hypoxemia alone is capable of inducing collateral vessels to form and that the novel gene Rabep2 is required.
Objective
Hypoxia stimulates angiogenesis during embryonic development and in pathological states. We hypothesized that hypoxia also stimulates collateral formation in adult heart by a process that involves RABEP2, a recently identified protein required for formation of collateral vessels during development.
Methods and results
Exposure of mice to reduced FiO2 induced collateral formation that resulted in smaller infarctions following LAD ligation and that reversed on return to normoxia. Deletion of Rabep2 or knockdown of Vegfa inhibited formation. Hypoxia upregulated Rabep2, Vegfa and Vegfr2 in heart and brain microvascular endothelial cells (HBMVECs). Knockdown of Rabep2 impaired migration of HBMVECs. In contrast to systemic hypoxia, deletion of Rabep2 did not affect collateral formation induced by ischemic injury caused by LAD ligation.
Conclusions
Hypoxia induced formation of coronary collaterals by a process that required VEGFA and RABEP2, proteins also required for collateral formation during development. Knockdown of Rabep2 impaired cell migration, providing one potential mechanism for RABEP2's role in collateral formation. This appears specific to hypoxia, since formation after acute ischemic injury was unaffected in Rabep2−/− mice. These findings provide a novel model for studying coronary collateral formation, and demonstrate that hypoxia alone can induce new collaterals to form in adult heart.
中文翻译:

吸入氧减少刺激成人心脏冠状动脉侧枝的从头形成
基本原理
当发生急性或慢性冠状动脉阻塞时,侧支血管可减轻心肌缺血。长期以来人们一直认为,尽管阻塞性疾病中原有的(预先存在的)络络增大,但成人中不会形成新的络络。然而,后者最近被证明是在冠状动脉结扎后发生的。由于直接研究侧支血管的困难以及信号通路的复杂环境(包括结扎诱导的细胞死亡),理解驱动这一过程的信号面临着挑战。在此,我们表明,仅低氧血症就能够诱导侧支血管形成,并且需要新的基因Rabep2 。
客观的
缺氧在胚胎发育和病理状态下刺激血管生成。我们假设缺氧还会通过涉及 RABEP2 的过程刺激成人心脏侧支血管的形成,RABEP2 是最近发现的一种在发育过程中形成侧支血管所需的蛋白质。
方法和结果
将小鼠暴露于减少的FiO 2诱导侧枝循环形成,导致LAD结扎后较小的梗塞,并在恢复正常含氧量后逆转。 Rabep2的缺失或Vegfa的敲低会抑制形成。缺氧上调心脏和脑微血管内皮细胞 (HBMVEC) 中的Rabep2 、 Vegfa和Vegfr2 。 Rabep2的敲低会损害 HBMVEC 的迁移。与全身性缺氧相反, Rabep2的缺失并不影响 LAD 结扎引起的缺血性损伤诱导的侧支循环形成。
结论
缺氧通过需要 VEGFA 和 RABEP2 的过程诱导冠状动脉侧枝循环的形成,这两种蛋白质也是发育过程中侧枝循环形成所必需的。 Rabep2的敲低会损害细胞迁移,为 RABEP2 在侧支形成中的作用提供了一种潜在机制。这似乎是缺氧所特有的,因为Rabep2 −/−小鼠急性缺血性损伤后的形成不受影响。这些发现为研究冠状动脉侧支形成提供了一种新的模型,并证明仅缺氧就可以诱导成人心脏中新的侧支形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号