Journal of Advanced Research ( IF 11.4 ) Pub Date : 2020-10-08 , DOI: 10.1016/j.jare.2020.09.009 Ulrike Breitinger 1, 2 , Kristina Weinländer 2 , Yvonne Pechmann 3 , Georg Langlhofer 4 , Ralf Enz 2 , Cord-Michael Becker 2 , Heinrich Sticht 2 , Matthias Kneussel 3 , Carmen Villmann 4 , Hans-Georg Breitinger 1, 2
|
Introduction
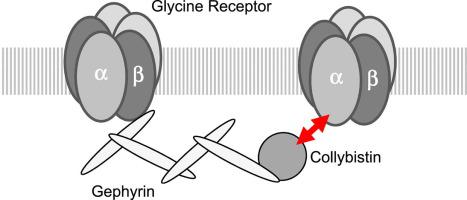
The inhibitory glycine receptor (GlyR), a mediator of fast synaptic inhibition, is located and held at neuronal synapses through the anchoring proteins gephyrin and collybistin. Stable localization of neurotransmitter receptors is essential for synaptic function. In case of GlyRs, only beta subunits were known until now to mediate synaptic anchoring.
Objectives
We identified a poly-proline II helix (PPII) in position 365–373 of the intra-cellular TM3-4 loop of the human GlyRα1 subunit as a novel potential synaptic anchoring site. The potential role of the PPII helix as synaptic anchoring site was tested.
Methods
Glycine receptors and collybistin variants were generated and recombinantly expressed in HEK293 cells and cultured neurons. Receptor function was assessed using patch-clamp electrophysiology, protein-protein interaction was studied using co-immuno-precipitation and pulldown experiments.
Results
Recombinantly expressed collybistin bound to isolated GlyRα1 TM3-4 loops in GST-pulldown assays. When the five proline residues P365A, P366A, P367A, P369A, P373A (GlyRα1P1-5A) located in the GlyRα1-PPII helix were replaced by alanines, the PPII secondary structure was disrupted. Recombinant GlyRα1P1-5A mutant subunits displayed normal cell surface expression and wildtype-like ion channel function, but binding to collybistin was abolished. The GlyRα1-collybistin interaction was independently confirmed by o-immunoprecipitation assays using full-length GlyRα1 subunits. Surprisingly, the interaction was not mediated by the SH3 domain of collybistin, but by its Pleckstrin homology (PH) domain. The mutation GlyRα1P366L, identified in a hyperekplexia patient, is also disrupting the PPII helix, and caused reduced collybistin binding.
Conclusion
Our data suggest a novel interaction between α1 GlyR subunits and collybistin, which is physiologically relevant in vitro and in vivo and may contribute to postsynaptic anchoring of glycine receptors.
中文翻译:

甘氨酸受体α1亚基细胞内大环中富含脯氨酸的基序与collybistin的Pleckstrin同源结构域相互作用
介绍
抑制性甘氨酸受体 (GlyR) 是快速突触抑制的介质,通过锚定蛋白 gephyrin 和 collybistin 定位并固定在神经元突触处。神经递质受体的稳定定位对于突触功能至关重要。在 GlyR 的情况下,直到现在只知道 β 亚基介导突触锚定。
目标
我们在人 GlyRα1 亚基的细胞内 TM3-4 环的第 365-373 位鉴定了聚脯氨酸 II 螺旋(PPII)作为新的潜在突触锚定位点。测试了 PPII 螺旋作为突触锚定位点的潜在作用。
方法
在 HEK293 细胞和培养的神经元中产生并重组表达甘氨酸受体和 collybistin 变体。使用膜片钳电生理学评估受体功能,使用免疫共沉淀和下拉实验研究蛋白质-蛋白质相互作用。
结果
在 GST-pulldown 测定中重组表达的 collybistin 与分离的 GlyRα1 TM3-4 环结合。当位于 GlyRα1-PPII 螺旋中的五个脯氨酸残基 P365A、P366A、P367A、P369A、P373A(GlyRα1 P1-5A)被丙氨酸取代时,PPII 二级结构被破坏。重组 GlyRα1 P1-5A突变亚基显示出正常的细胞表面表达和类似野生型的离子通道功能,但与 collybistin 的结合被取消。GlyRα1-collybistin 相互作用通过使用全长 GlyRα1 亚基的 o-免疫沉淀测定独立证实。令人惊讶的是,这种相互作用不是由 collybistin 的 SH3 域介导的,而是由其 Pleckstrin 同源 (PH) 域介导的。突变 GlyRα1 P366L, 在一个过度焦虑症患者中发现, 也破坏了 PPII 螺旋, 并导致 collybistin 结合减少。
结论
我们的数据表明 α1 GlyR 亚基和 collybistin 之间存在新的相互作用,这在体外和体内具有生理相关性,并且可能有助于甘氨酸受体的突触后锚定。











































 京公网安备 11010802027423号
京公网安备 11010802027423号