Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A point-of-care selenium nanoparticle-based test for the combined detection of anti-SARS-CoV-2 IgM and IgG in human serum and blood
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-10-07 , DOI: 10.1039/d0lc00828a Zhizeng Wang 1, 2, 3, 4, 5 , Zhi Zheng 1, 2, 3, 4, 5 , Hangzhan Hu 1, 2, 3, 4, 5 , Qianwen Zhou 1, 2, 3, 4, 5 , Wei Liu 6, 7, 8 , Xiaoquan Li 5, 8, 9 , Zhigang Liu 5, 8, 9 , Yaohui Wang 1, 2, 3, 4, 5 , Yuanfang Ma 1, 2, 3, 4, 5
Lab on a Chip ( IF 6.1 ) Pub Date : 2020-10-07 , DOI: 10.1039/d0lc00828a Zhizeng Wang 1, 2, 3, 4, 5 , Zhi Zheng 1, 2, 3, 4, 5 , Hangzhan Hu 1, 2, 3, 4, 5 , Qianwen Zhou 1, 2, 3, 4, 5 , Wei Liu 6, 7, 8 , Xiaoquan Li 5, 8, 9 , Zhigang Liu 5, 8, 9 , Yaohui Wang 1, 2, 3, 4, 5 , Yuanfang Ma 1, 2, 3, 4, 5
Affiliation
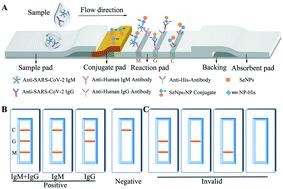
|
COVID-19 is a widespread and highly contagious disease in the human population. COVID-19 is caused by SARS-CoV-2 infection. There is still a great demand for point-of-care tests for detection, epidemic prevention and epidemiological investigation, both now and after the epidemic. We present a lateral flow immunoassay kit based on a selenium nanoparticle-modified SARS-CoV-2 nucleoprotein, which detects anti-SARS-CoV-2 IgM and anti-SARS-CoV-2 IgG in human serum, and the results can be read by the naked eye in 10 minutes. We expressed and purified the SARS-CoV-2 nucleoprotein in HEK293 cells, with a purity of 98.14% and a concentration of 5 mg mL−1. Selenium nanoparticles were synthesized by L-ascorbic acid reduction of seleninic acid at room temperature. After conjugation with the nucleoprotein, a lateral flow kit was successfully prepared. The IgM and IgG detection limits of the lateral flow kit reached 20 ng mL−1 and 5 ng mL−1, respectively, in human serum. A clinical study sample comprising 90 COVID-19-diagnosed patients and 263 non-infected controls was used to demonstrate a sensitivity and specificity of 93.33% and 97.34%, respectively, based on RT-PCR and clinical results. No cross-reactions with rheumatoid factor and positive serum for anti-nuclear antibodies, influenza A, and influenza B were observed. Moreover, the lateral flow kit remained stable after storage for 30 days at 37 °C. Our results demonstrate that the selenium nanoparticle lateral flow kit can conveniently, rapidly, and sensitively detect anti-SARS-CoV-2 IgM and IgG in human serum and blood; it can also be suitable for the epidemiological investigation of COVID-19.
中文翻译:

基于护理点硒纳米粒子的测试,用于组合检测人血清和血液中的抗SARS-CoV-2 IgM和IgG
COVID-19是人类中一种广泛传播且高度传染的疾病。COVID-19是由SARS-CoV-2感染引起的。在现在和之后,仍然存在对用于检测,防疫和流行病学调查的即时检验的巨大需求。我们提出一种基于硒纳米粒子修饰的SARS-CoV-2核蛋白的侧向流免疫分析试剂盒,该试剂盒可检测人血清中的抗SARS-CoV-2 IgM和抗SARS-CoV-2 IgG,其结果可读取用肉眼在10分钟内。我们在HEK293细胞中表达和纯化了SARS-CoV-2核蛋白,纯度为98.14%,浓度为5 mg mL -1。硒纳米粒子由合成大号-抗坏血酸在室温下还原硒酸。与核蛋白结合后,成功制备了侧流试剂盒。横向流动试剂盒的IgM和IgG检测极限分别达到20 ng mL -1和5 ng mL -1分别在人血清中。根据RT-PCR和临床结果,使用包含90位经COVID-19诊断的患者和263位未感染对照的临床研究样本,证明其敏感性和特异性分别为93.33%和97.34%。没有观察到与类风湿因子和阳性血清抗核抗体,甲型流感和乙型流感的交叉反应。此外,侧向流动套件在37°C下储存30天后仍保持稳定。我们的结果表明,硒纳米粒子侧向流动试剂盒可以方便,快速,灵敏地检测人血清和血液中的抗SARS-CoV-2 IgM和IgG。它也可能适合于COVID-19的流行病学调查。
更新日期:2020-11-03
中文翻译:

基于护理点硒纳米粒子的测试,用于组合检测人血清和血液中的抗SARS-CoV-2 IgM和IgG
COVID-19是人类中一种广泛传播且高度传染的疾病。COVID-19是由SARS-CoV-2感染引起的。在现在和之后,仍然存在对用于检测,防疫和流行病学调查的即时检验的巨大需求。我们提出一种基于硒纳米粒子修饰的SARS-CoV-2核蛋白的侧向流免疫分析试剂盒,该试剂盒可检测人血清中的抗SARS-CoV-2 IgM和抗SARS-CoV-2 IgG,其结果可读取用肉眼在10分钟内。我们在HEK293细胞中表达和纯化了SARS-CoV-2核蛋白,纯度为98.14%,浓度为5 mg mL -1。硒纳米粒子由合成大号-抗坏血酸在室温下还原硒酸。与核蛋白结合后,成功制备了侧流试剂盒。横向流动试剂盒的IgM和IgG检测极限分别达到20 ng mL -1和5 ng mL -1分别在人血清中。根据RT-PCR和临床结果,使用包含90位经COVID-19诊断的患者和263位未感染对照的临床研究样本,证明其敏感性和特异性分别为93.33%和97.34%。没有观察到与类风湿因子和阳性血清抗核抗体,甲型流感和乙型流感的交叉反应。此外,侧向流动套件在37°C下储存30天后仍保持稳定。我们的结果表明,硒纳米粒子侧向流动试剂盒可以方便,快速,灵敏地检测人血清和血液中的抗SARS-CoV-2 IgM和IgG。它也可能适合于COVID-19的流行病学调查。



























 京公网安备 11010802027423号
京公网安备 11010802027423号