当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The simplest Diels–Alder reactions are not endo-selective
Chemical Science ( IF 7.6 ) Pub Date : 2020-10-06 , DOI: 10.1039/d0sc04553e William J Lording 1 , Thomas Fallon 1 , Michael S Sherburn 1 , Michael N Paddon-Row 2
Chemical Science ( IF 7.6 ) Pub Date : 2020-10-06 , DOI: 10.1039/d0sc04553e William J Lording 1 , Thomas Fallon 1 , Michael S Sherburn 1 , Michael N Paddon-Row 2
Affiliation
|
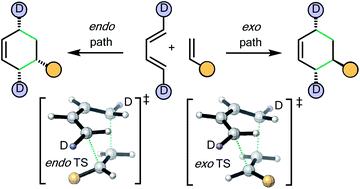
There is a widespread perception that the high level of endo selectivity witnessed in many Diels–Alder reactions is an intrinsic feature of the transformation. In contrast to expectations based upon this existing belief, the first experimental Diels–Alder reactions of a novel, deuterium-labeled 1,3-butadiene with commonly used mono-substituted alkenic dienophiles (acrolein, methyl vinyl ketone, acrylic acid, methyl acrylate, acrylamide and acrylonitrile) reveal kinetic endo : exo ratios close to 1 : 1. Maleonitrile, butenolide, α-methylene γ-butyrolactone, and N-methylmaleimide behave differently, as does methyl vinyl ketone under Lewis acid catalysis. CBS-QB3 calculations incorporating solvent and temperature parameters give endo : exo product ratios that are in near quantitative agreement with these and earlier experimental findings. This work challenges the preconception of innate endo-selectivity by providing the first experimental evidence that the simplest Diels–Alder reactions are not endo-selective. Trends in behaviour are traced to steric and electronic effects in Diels–Alder transition structures, giving new insights into these fundamental processes.
中文翻译:

最简单的狄尔斯-阿尔德反应不是内选择性的
人们普遍认为,许多狄尔斯-阿尔德反应中的高水平内选择性是该转化的内在特征。与基于这一现有信念的预期相反,新型氘标记的 1,3-丁二烯与常用的单取代烯基双烯体(丙烯醛、甲基乙烯基酮、丙烯酸、丙烯酸甲酯、丙烯酰胺和丙烯腈)揭示了动力学内型 : 外型比率接近 1:1。顺丁烯二腈、丁烯内酯、α-亚甲基、γ-丁内酯和N-甲基马来酰亚胺的行为不同,甲基乙烯基酮在路易斯酸催化下也是如此。结合溶剂和温度参数的 CBS-QB3 计算得出的内型 : 外型产物比率与这些和早期的实验结果在数量上几乎一致。这项工作通过提供第一个实验证据证明最简单的狄尔斯-阿尔德反应不是内选择性的,挑战了先天内选择性的先入之见。行为趋势可追溯到第尔斯-阿尔德转变结构中的空间和电子效应,为这些基本过程提供了新的见解。
更新日期:2020-10-15
中文翻译:

最简单的狄尔斯-阿尔德反应不是内选择性的
人们普遍认为,许多狄尔斯-阿尔德反应中的高水平内选择性是该转化的内在特征。与基于这一现有信念的预期相反,新型氘标记的 1,3-丁二烯与常用的单取代烯基双烯体(丙烯醛、甲基乙烯基酮、丙烯酸、丙烯酸甲酯、丙烯酰胺和丙烯腈)揭示了动力学内型 : 外型比率接近 1:1。顺丁烯二腈、丁烯内酯、α-亚甲基、γ-丁内酯和N-甲基马来酰亚胺的行为不同,甲基乙烯基酮在路易斯酸催化下也是如此。结合溶剂和温度参数的 CBS-QB3 计算得出的内型 : 外型产物比率与这些和早期的实验结果在数量上几乎一致。这项工作通过提供第一个实验证据证明最简单的狄尔斯-阿尔德反应不是内选择性的,挑战了先天内选择性的先入之见。行为趋势可追溯到第尔斯-阿尔德转变结构中的空间和电子效应,为这些基本过程提供了新的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号