当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Process Development of Sotagliflozin, a Dual Inhibitor of Sodium–Glucose Cotransporter-1/2 for the Treatment of Diabetes
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-10-07 , DOI: 10.1021/acs.oprd.0c00359 Matthew M. Zhao 1 , Haiming Zhang 1 , Shinya Iimura 1 , Mark S. Bednarz 1 , Qiu-Ling Song 1 , Ngiap-Kie Lim 1 , Jie Yan 1 , Wenxue Wu 1 , Kuangchu Dai 2 , Xiaodong Gu 2 , Youchu Wang 2
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-10-07 , DOI: 10.1021/acs.oprd.0c00359 Matthew M. Zhao 1 , Haiming Zhang 1 , Shinya Iimura 1 , Mark S. Bednarz 1 , Qiu-Ling Song 1 , Ngiap-Kie Lim 1 , Jie Yan 1 , Wenxue Wu 1 , Kuangchu Dai 2 , Xiaodong Gu 2 , Youchu Wang 2
Affiliation
|
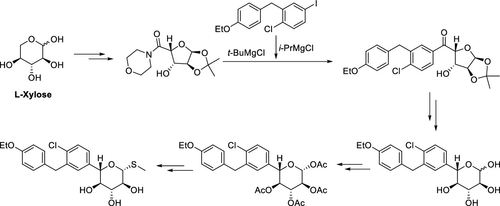
The development of an efficient manufacturing process for sotagliflozin (LX4211), a dual inhibitor of sodium–glucose cotransporter-1/2 (SGLT-1/2) for the treatment of diabetes, is described. Sotagliflozin features five contiguous chiral centers on the carbohydrate core flanked by a thioether group and a biaryl moiety. Three chiral centers are obtained from the starting material l-xylose, while the other two were established (or modified) via three highly stereoselective transformations: Luche reduction (dr: 97/3), dynamic kinetic resolution of anomeric hemiacetal (dr: 95/5), and Lewis acid-promoted thiolation (dr: 1000/1). Global deprotection of the resulting penultimate intermediate with catalytic sodium methoxide followed by recrystallization furnishes sotagliflozin. The longest linear sequence consists of 10 steps from l-xylose with an overall yield of 40%. This process has been performed on multi-hundred kilogram batches to satisfy the drug substance development demands.
中文翻译:

钠-葡萄糖共转运蛋白-1/2双重抑制剂索格列净的工艺开发
描述了有效的制造工艺索格列净(LX4211)的研究,索格列净是钠-葡萄糖共转运蛋白1/2的双重抑制剂(SGLT-1 / 2)用于糖尿病的治疗。Sotagliflozin在碳水化合物核心上具有五个连续的手性中心,两侧是硫醚基和联芳基部分。从原料1-木糖获得三个手性中心,而另外两个则通过三个高度立体选择性的转化:Luche还原(dr:97/3),异头半缩醛的动态动力学拆分(dr:95/5)和Lewis酸促进的硫醇化(dr:1000/1)。用催化的甲醇钠对所得的倒数第二个中间体进行整体脱保护,然后重结晶,从而提供了索格列净。最长的线性序列由1-木糖形成10步,总产率为40%。此过程已针对数百公斤批次执行,以满足原料药开发需求。
更新日期:2020-11-21
中文翻译:

钠-葡萄糖共转运蛋白-1/2双重抑制剂索格列净的工艺开发
描述了有效的制造工艺索格列净(LX4211)的研究,索格列净是钠-葡萄糖共转运蛋白1/2的双重抑制剂(SGLT-1 / 2)用于糖尿病的治疗。Sotagliflozin在碳水化合物核心上具有五个连续的手性中心,两侧是硫醚基和联芳基部分。从原料1-木糖获得三个手性中心,而另外两个则通过三个高度立体选择性的转化:Luche还原(dr:97/3),异头半缩醛的动态动力学拆分(dr:95/5)和Lewis酸促进的硫醇化(dr:1000/1)。用催化的甲醇钠对所得的倒数第二个中间体进行整体脱保护,然后重结晶,从而提供了索格列净。最长的线性序列由1-木糖形成10步,总产率为40%。此过程已针对数百公斤批次执行,以满足原料药开发需求。



























 京公网安备 11010802027423号
京公网安备 11010802027423号