当前位置:
X-MOL 学术
›
Helv. Chimica Acta
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total Synthesis of (−)‐Rotundone and (−)‐epi‐Rotundone from Monoterpene Precursors
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2020-10-06 , DOI: 10.1002/hlca.202000129 Fridtjof Schröder 1 , Fabian Rüthi 2
Helvetica Chimica Acta ( IF 1.5 ) Pub Date : 2020-10-06 , DOI: 10.1002/hlca.202000129 Fridtjof Schröder 1 , Fabian Rüthi 2
Affiliation
|
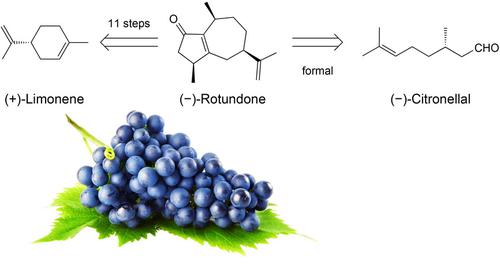
The first total synthesis of (−)‐rotundone has been accomplished from (+)‐(R)‐limonene and therefore for the first time from an unrelated monoterpene instead of modifying structurally closely related sesquiterpene precursors such as α‐guaiene. Challenges such as intermediates with stereocenters prone to epimerization by enolization were overcome by designing a β‐methyl‐keto route starting from (+)‐(R)‐limonene which finally gave (−)‐rotundone by Nazarov cyclization of a precursor 13a. Diastereomer (−)‐epi‐rotundone was separated from (−)‐rotundone chromatographically. An alternative route from rac‐citronellal provided a diastereomer mixture of racemic Nazarov precursor 13 through a TRIP‐catalyzed intramolecular aldolization, thus indicating that the Nazarov cyclization precursor 13a is in principle accessible from (−)‐(S)‐citronellal. The 11‐step synthesis from (+)‐(R)‐limonene with ca. 1 % overall yield confirmed the absolute configuration of (−)‐rotundone and provided samples of good olfactory quality.
中文翻译:

单萜前体的全合成(-)-酮体和(-)-表酮体
(-)-雄酮的首次全合成是由(+)-(R)-柠檬烯完成的,因此是第一次从不相关的单萜烯开始,而不是修饰结构紧密相关的倍半萜烯前体,例如α-番石榴烯。通过设计从(+)-(R)-柠檬烯开始的β-甲基-酮基途径克服了诸如立体中心易于通过烯醇化来异构化等挑战,该途径最终通过前体13a的Nazarov环化获得了(-)-雄酮。非对映体(-)- Epi -rotundone色谱分离与(-)-rotundone色谱分离。rac- citronellal的另一种方法提供了外消旋纳扎罗夫的非对映异构体混合物前体13通过TRIP催化的分子内醛醇缩合,因此表明Nazarov环化前体13a原则上可从(-)-(S)-香茅醛中获得。由(+)-(R)-柠檬烯的ca进行11步合成。1%的总收率证实了(-)-酮的绝对构型,并提供了嗅觉良好的样品。
更新日期:2020-11-25
中文翻译:

单萜前体的全合成(-)-酮体和(-)-表酮体
(-)-雄酮的首次全合成是由(+)-(R)-柠檬烯完成的,因此是第一次从不相关的单萜烯开始,而不是修饰结构紧密相关的倍半萜烯前体,例如α-番石榴烯。通过设计从(+)-(R)-柠檬烯开始的β-甲基-酮基途径克服了诸如立体中心易于通过烯醇化来异构化等挑战,该途径最终通过前体13a的Nazarov环化获得了(-)-雄酮。非对映体(-)- Epi -rotundone色谱分离与(-)-rotundone色谱分离。rac- citronellal的另一种方法提供了外消旋纳扎罗夫的非对映异构体混合物前体13通过TRIP催化的分子内醛醇缩合,因此表明Nazarov环化前体13a原则上可从(-)-(S)-香茅醛中获得。由(+)-(R)-柠檬烯的ca进行11步合成。1%的总收率证实了(-)-酮的绝对构型,并提供了嗅觉良好的样品。











































 京公网安备 11010802027423号
京公网安备 11010802027423号