当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
N−N Bond Formation Using an Iodonitrene as an Umpolung of Ammonia: Straightforward and Chemoselective Synthesis of Hydrazinium Salts
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-06 , DOI: 10.1002/adsc.202001047 Arianna Tota 1 , Marco Colella 1 , Claudia Carlucci 1 , Andrea Aramini 2 , Guy Clarkson 3 , Leonardo Degennaro 1 , James Bull 4 , Renzo Luisi 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-06 , DOI: 10.1002/adsc.202001047 Arianna Tota 1 , Marco Colella 1 , Claudia Carlucci 1 , Andrea Aramini 2 , Guy Clarkson 3 , Leonardo Degennaro 1 , James Bull 4 , Renzo Luisi 1
Affiliation
|
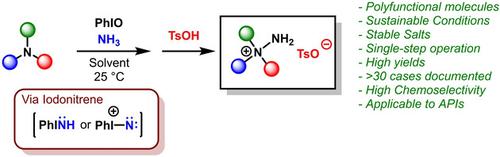
The formation of hydrazinium salts by N−N bond formation has typically involved the use of hazardous and difficult to handle reagents. Here, mild and operationally simple conditions for the synthesis of hydrazinium salts are reported. Electrophilic nitrogen transfer to the nitrogen atom of tertiary amines is achieved using iodosylbenzene as oxidant and ammonium carbamate as the N‐source. The resulting process is highly chemoselective and tolerant to other functional groups. A wide scope is reported, including examples with bioactive molecules. Insights on the structure of hydrazinium salts were provided by X‐ray analysis.
中文翻译:

使用碘代丁烯作为氨的N-N键形成:dra盐的直接合成和化学选择性合成
通过N–N键形成肼盐通常涉及使用危险且难以处理的试剂。在此,报道了合成肼盐的温和且操作简单的条件。亲电子转移到叔胺的氮原子上是通过碘烷基苯作为氧化剂和氨基甲酸铵作为N源实现的。所产生的过程是高度化学选择性的,并且对其他官能团具有耐受性。据报道范围很广,包括具有生物活性分子的例子。X射线分析提供了有关肼盐结构的见解。
更新日期:2020-10-06
中文翻译:

使用碘代丁烯作为氨的N-N键形成:dra盐的直接合成和化学选择性合成
通过N–N键形成肼盐通常涉及使用危险且难以处理的试剂。在此,报道了合成肼盐的温和且操作简单的条件。亲电子转移到叔胺的氮原子上是通过碘烷基苯作为氧化剂和氨基甲酸铵作为N源实现的。所产生的过程是高度化学选择性的,并且对其他官能团具有耐受性。据报道范围很广,包括具有生物活性分子的例子。X射线分析提供了有关肼盐结构的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号