当前位置:
X-MOL 学术
›
Chem. Biodivers.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cajuputones A–C, β‐triketone flavanone hybrids from the branches and leaves of Melaleuca cajuputi
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-10-27 , DOI: 10.1002/cbdv.202000706 Wen-Jun Xu 1 , Xin Xie 1 , Lin Wu 1 , Xiao-Meng Tian 1 , Cheng-Cheng Wang 2 , Ling-Yi Kong 3 , Jun Luo 1
Chemistry & Biodiversity ( IF 2.3 ) Pub Date : 2020-10-27 , DOI: 10.1002/cbdv.202000706 Wen-Jun Xu 1 , Xin Xie 1 , Lin Wu 1 , Xiao-Meng Tian 1 , Cheng-Cheng Wang 2 , Ling-Yi Kong 3 , Jun Luo 1
Affiliation
|
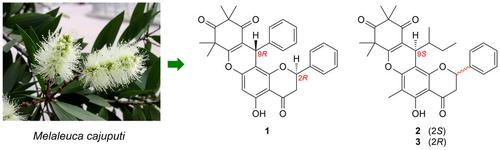
Three new β‐triketone flavanone hybrids, cajuputones A–C were obtained from Melaleuca cajuputi (the Australian ‘tea tree’). The structures of cajuputones A–C were elucidated by 1D/2D NMR spectroscopy and HR‐ESI‐MS analyses; and their absolute configurations were established by electric circular dichroism (ECD) calculations using TDDFT method. Structurally, cajuputones A–C feature a rare 6/6/6/6 oxatetracyclic ring system fused between an acylphloroglucinol‐derived β‐triketone and a pinocembrin or strobopinin moiety via an angle‐type pyran‐like motif. DFT‐based conformational optimization in chloroform explained the similarity of the 1D NMR data of cajuputones B and C (C‐2 epimers).
中文翻译:

Cajuputones A-C, 来自白千层枝叶的β-三酮黄烷酮杂合体
从 Melaleuca cajuputi(澳大利亚“茶树”)中获得了三种新的 β-三酮黄烷酮杂种 cajuputones A-C。cajuputones A-C的结构通过1D/2D NMR光谱和HR-ESI-MS分析得到阐明;并且它们的绝对构型是通过使用 TDDFT 方法通过电圆二色性 (ECD) 计算建立的。在结构上,cajuputones A-C 具有罕见的 6/6/6/6 氧杂四环系统,通过角型吡喃样基序在酰基间苯三酚衍生的 β-三酮和松香蛋白或 strobopinin 部分之间融合。氯仿中基于 DFT 的构象优化解释了 cajuputones B 和 C(C-2 差向异构体)的 1D NMR 数据的相似性。
更新日期:2020-10-27
中文翻译:

Cajuputones A-C, 来自白千层枝叶的β-三酮黄烷酮杂合体
从 Melaleuca cajuputi(澳大利亚“茶树”)中获得了三种新的 β-三酮黄烷酮杂种 cajuputones A-C。cajuputones A-C的结构通过1D/2D NMR光谱和HR-ESI-MS分析得到阐明;并且它们的绝对构型是通过使用 TDDFT 方法通过电圆二色性 (ECD) 计算建立的。在结构上,cajuputones A-C 具有罕见的 6/6/6/6 氧杂四环系统,通过角型吡喃样基序在酰基间苯三酚衍生的 β-三酮和松香蛋白或 strobopinin 部分之间融合。氯仿中基于 DFT 的构象优化解释了 cajuputones B 和 C(C-2 差向异构体)的 1D NMR 数据的相似性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号