当前位置:
X-MOL 学术
›
Biofactors
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of photostability and phototoxicity of esterified derivatives of ubiquinol‐10 and their application as prodrugs of reduced coenzyme Q10 for topical administration
Biofactors ( IF 5.0 ) Pub Date : 2020-10-06 , DOI: 10.1002/biof.1678 Shuichi Setoguchi 1 , Nami Nagata-Akaho 1 , Shotaro Goto 1 , Hirofumi Yamakawa 1 , Daisuke Watase 1 , Kazuki Terada 1 , Mitsuhisa Koga 1 , Kazuhisa Matsunaga 1 , Yoshiharu Karube 1 , Jiro Takata 1
Biofactors ( IF 5.0 ) Pub Date : 2020-10-06 , DOI: 10.1002/biof.1678 Shuichi Setoguchi 1 , Nami Nagata-Akaho 1 , Shotaro Goto 1 , Hirofumi Yamakawa 1 , Daisuke Watase 1 , Kazuki Terada 1 , Mitsuhisa Koga 1 , Kazuhisa Matsunaga 1 , Yoshiharu Karube 1 , Jiro Takata 1
Affiliation
|
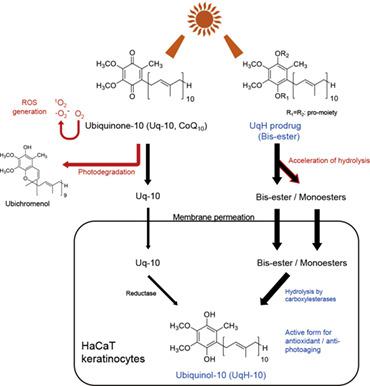
Ubiquinol‐10 (UqH‐10), the fully reduced form of ubiquinone‐10 (Uq‐10, coenzyme Q10), is an antioxidant and is involved in energy production. However, physicochemical disadvantages, such as rapid oxidation, water‐insolubility, photoinstability, and phototoxicity, limit its application. We previously reported that UqH‐10 1,4‐bis‐N,N‐dimethylglycinate improved the oxidation susceptibility and poor bioavailability of UqH‐10 in rats. Herein, we evaluated the photochemical properties of UqH‐esterified derivatives (N,N‐dimethylglycinate, hemi‐succinate, ethylsuccinate, and hemi‐glutarate). Photostability was examined by irradiation using artificial sunlight and monochromatic light. The concentration of each compound was determined using LC–MS/MS. Phototoxicity was assessed by singlet oxygen and superoxide assays. Delivery of UqH‐10 via UqH‐esters to the HaCaT human keratinocyte cell line was determined using LC–MS/MS. UqH‐esters showed higher photostability to artificial sunlight than Uq‐10 and UqH‐10. Uq‐10 and UqH‐10 were rapidly degraded by monochromatic light at 279 nm, whereas UqH‐esters were more stable. UVA and/or UVB irradiation generated high levels of singlet oxygen and superoxide in Uq‐10, whereas UqH‐esters were unreactive. Additionally, UqH‐esters effectively delivered UqH‐10 to HaCaT cells following efficient uptake in their ester forms and ester bond hydrolysis in the cells. In conclusion, UqH‐ester derivatives exhibit higher photostability and lower phototoxicity compared with Uq‐10 and UqH‐10.
中文翻译:

泛醇-10酯化衍生物的光稳定性和光毒性评价及其作为还原型辅酶Q10前药局部给药的应用
Ubiquinol-10 (UqH-10) 是 ubiquinone-10 (Uq-10, coenzyme Q 10 )的完全还原形式,是一种抗氧化剂,参与能量产生。然而,快速氧化、不溶于水、光不稳定性和光毒性等物理化学缺点限制了其应用。我们之前报道过 UqH-10 1,4-双-N,N-二甲基甘氨酸盐改善了大鼠中 UqH-10 的氧化敏感性和较差的生物利用度。在此,我们评估了 UqH 酯化衍生物 ( N,N-二甲基甘氨酸、半琥珀酸、乙基琥珀酸和半戊二酸)。通过使用人造阳光和单色光的照射来检查光稳定性。使用 LC-MS/MS 确定每种化合物的浓度。通过单线态氧和超氧化物测定评估光毒性。使用 LC-MS/MS 测定通过 UqH 酯向 HaCaT 人角质形成细胞系输送 UqH-10。与 Uq-10 和 UqH-10 相比,UqH-酯对人造阳光表现出更高的光稳定性。Uq-10 和 UqH-10 被 279 nm 的单色光迅速降解,而 UqH-酯更稳定。UVA 和/或 UVB 辐照在 Uq-10 中产生高水平的单线态氧和超氧化物,而 UqH-酯不反应。此外,UqH-酯有效地将 UqH-10 传递给 HaCaT 细胞,因为它们的酯形式被有效吸收并在细胞中水解了酯键。总之,与Uq-10和UqH-10相比,UqH-酯衍生物表现出更高的光稳定性和更低的光毒性。
更新日期:2020-10-06
中文翻译:

泛醇-10酯化衍生物的光稳定性和光毒性评价及其作为还原型辅酶Q10前药局部给药的应用
Ubiquinol-10 (UqH-10) 是 ubiquinone-10 (Uq-10, coenzyme Q 10 )的完全还原形式,是一种抗氧化剂,参与能量产生。然而,快速氧化、不溶于水、光不稳定性和光毒性等物理化学缺点限制了其应用。我们之前报道过 UqH-10 1,4-双-N,N-二甲基甘氨酸盐改善了大鼠中 UqH-10 的氧化敏感性和较差的生物利用度。在此,我们评估了 UqH 酯化衍生物 ( N,N-二甲基甘氨酸、半琥珀酸、乙基琥珀酸和半戊二酸)。通过使用人造阳光和单色光的照射来检查光稳定性。使用 LC-MS/MS 确定每种化合物的浓度。通过单线态氧和超氧化物测定评估光毒性。使用 LC-MS/MS 测定通过 UqH 酯向 HaCaT 人角质形成细胞系输送 UqH-10。与 Uq-10 和 UqH-10 相比,UqH-酯对人造阳光表现出更高的光稳定性。Uq-10 和 UqH-10 被 279 nm 的单色光迅速降解,而 UqH-酯更稳定。UVA 和/或 UVB 辐照在 Uq-10 中产生高水平的单线态氧和超氧化物,而 UqH-酯不反应。此外,UqH-酯有效地将 UqH-10 传递给 HaCaT 细胞,因为它们的酯形式被有效吸收并在细胞中水解了酯键。总之,与Uq-10和UqH-10相比,UqH-酯衍生物表现出更高的光稳定性和更低的光毒性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号