当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reactivity, Selectivity, and Synthesis of 4‐C‐Silylated Glycosyl Donors and 4‐Deoxy Analogues
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-07 , DOI: 10.1002/anie.202009209 Martin Jæger Pedersen 1 , Christian Marcus Pedersen 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-07 , DOI: 10.1002/anie.202009209 Martin Jæger Pedersen 1 , Christian Marcus Pedersen 2
Affiliation
|
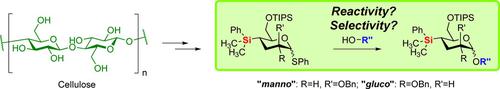
A method for introducing dimethylphenylsilyl at the 4‐position in carbohydrates has been developed. Two C‐silylated glycosyl donors were prepared via levoglucosenone, starting from cellulose. The glycosylation properties were studied using three glucoside acceptors, a 3‐OH, 4‐OH, and 6‐OH. Compared with the 4‐deoxy variant, it was found that the anomeric selectivity was influenced more by the C‐2 substituents orientation than the silyl in the 4‐position. In general, the reactivity of these donors was higher than the corresponding 4‐deoxy‐analogue, albeit a competition experiment showed that the introduction of a C−Si increases the relative reactivity by a modest factor of around two.
中文翻译:

4 C甲硅烷基化糖基供体和4脱氧类似物的反应性,选择性和合成
已经开发了一种在碳水化合物的4位引入二甲基苯基甲硅烷基的方法。从纤维素开始,通过左旋葡糖酮制备了两个C-甲硅烷基化的糖基供体。使用三个葡糖苷受体(3-OH,4-OH和6-OH)研究了糖基化性质。与4-脱氧变体相比,发现异头异构体选择性受C-2取代基取向的影响要大于4-位的甲硅烷基。通常,这些供体的反应性高于相应的4-脱氧类似物,尽管竞争实验表明,引入C-Si可使相对反应性增加约2的适度因素。
更新日期:2020-10-07
中文翻译:

4 C甲硅烷基化糖基供体和4脱氧类似物的反应性,选择性和合成
已经开发了一种在碳水化合物的4位引入二甲基苯基甲硅烷基的方法。从纤维素开始,通过左旋葡糖酮制备了两个C-甲硅烷基化的糖基供体。使用三个葡糖苷受体(3-OH,4-OH和6-OH)研究了糖基化性质。与4-脱氧变体相比,发现异头异构体选择性受C-2取代基取向的影响要大于4-位的甲硅烷基。通常,这些供体的反应性高于相应的4-脱氧类似物,尽管竞争实验表明,引入C-Si可使相对反应性增加约2的适度因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号