当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Decarboxylative C−N Formation to Generate Alkyl, Alkenyl, and Aryl Amines
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-07 , DOI: 10.1002/anie.202010974 Yipin Zhang 1 , Xia Ge 1 , Hongjian Lu 2 , Guigen Li 3
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-10-07 , DOI: 10.1002/anie.202010974 Yipin Zhang 1 , Xia Ge 1 , Hongjian Lu 2 , Guigen Li 3
Affiliation
|
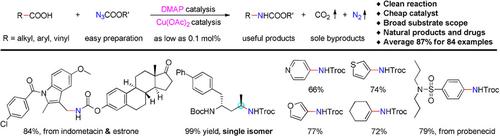
Transition‐metal‐catalyzed sp2 C−N bond formation is a reliable method for the synthesis of aryl amines. Catalytic sp3 C−N formation reactions have been reported occasionally, and methods that can realize both sp2 and sp3 C−N formation are relatively unexplored. Herein, we address this challenge with a method of catalytic decarboxylative C−N formation that proceeds through a cascade carboxylic acid activation, acyl azide formation, Curtius rearrangement and nucleophilic addition reaction. The reaction uses naturally abundant organic carboxylic acids as carbon sources, readily prepared azidoformates as the nitrogen sources, and 4‐dimethylaminopyridine (DMAP) and Cu(OAc)2 as catalysts with as low as 0.1 mol % loading, providing protected alkyl, alkenyl and aryl amines in high yields with gaseous N2 and CO2 as the only byproducts. Examples are demonstrated of the late‐stage functionalization of natural products and drug molecules, stereospecific synthesis of useful α‐chiral alkyl amines, and rapid construction of different ureas and primary amines.
中文翻译:

催化脱羧CN生成以生成烷基,烯基和芳基胺
过渡金属催化的sp 2 C-N键形成是合成芳基胺的可靠方法。偶尔已经报道了催化sp 3 C-N的形成反应,并且相对未开发能够同时实现sp 2和sp 3 C-N形成的方法。在这里,我们通过催化脱羧CN的形成方法来解决这一挑战,该方法通过级联羧酸活化,酰基叠氮化物形成,Curtius重排和亲核加成反应进行。该反应使用天然丰富的有机羧酸作为碳源,容易制备的叠氮基甲酸作为氮源,以及4-二甲基氨基吡啶(DMAP)和Cu(OAc)2作为负载量低至0.1 mol%的催化剂,以气态N 2和CO 2为唯一副产物,可以高收率提供受保护的烷基,烯基和芳基胺。实例展示了天然产物和药物分子的后期功能化,有用的α-手性烷基胺的立体定向合成以及不同尿素和伯胺的快速构建。
更新日期:2020-10-07
中文翻译:

催化脱羧CN生成以生成烷基,烯基和芳基胺
过渡金属催化的sp 2 C-N键形成是合成芳基胺的可靠方法。偶尔已经报道了催化sp 3 C-N的形成反应,并且相对未开发能够同时实现sp 2和sp 3 C-N形成的方法。在这里,我们通过催化脱羧CN的形成方法来解决这一挑战,该方法通过级联羧酸活化,酰基叠氮化物形成,Curtius重排和亲核加成反应进行。该反应使用天然丰富的有机羧酸作为碳源,容易制备的叠氮基甲酸作为氮源,以及4-二甲基氨基吡啶(DMAP)和Cu(OAc)2作为负载量低至0.1 mol%的催化剂,以气态N 2和CO 2为唯一副产物,可以高收率提供受保护的烷基,烯基和芳基胺。实例展示了天然产物和药物分子的后期功能化,有用的α-手性烷基胺的立体定向合成以及不同尿素和伯胺的快速构建。











































 京公网安备 11010802027423号
京公网安备 11010802027423号