Journal of Proteomics ( IF 2.8 ) Pub Date : 2020-10-07 , DOI: 10.1016/j.jprot.2020.104005 Yuki Okawara 1 , Hisashi Hirano 1 , Ayuko Kimura 1 , Natsumi Sato 1 , Yuriko Hayashi 1 , Makoto Osada 1 , Takao Kawakami 2 , Norihisa Ootake 3 , Eiji Kinoshita 4 , Kiyotaka Fujita 1
|
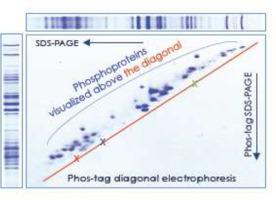
Phos-tag diagonal electrophoresis was developed to identify precisely a change in electrophoretic mobility of phosphoproteins in Phos-tag SDS-PAGE. Previously, if a single protein band was detected, it was impossible to determine whether mobility of the protein altered by Mn2+ Phos-tag in Phos-tag SDS-PAGE gels because SDS-PAGE and Phos-tag SDS-PAGE were performed on different gels. Moreover, when multiple protein bands were detected, it was difficult to determine whether the band with the highest mobility was altered mobility by Mn2+ Phos-tag. However, these problems were resolved by Phos-tag diagonal electrophoresis in which SDS-PAGE and Phos-tag SDS-PAGE patterns were provided on a single gel. Using this technique we identified phosphorylation states of various proteins such as α-lactalbumin, α- and β-casein, ovalbumin, basic 7S globulin, and 26S proteasome subunits. In the analyses of 26S proteasome subunits from humans and yeast, we could confirm that all subunits are phosphorylated, and find that the number of major proteins with different phosphorylation states is a few in each of the subunits despite having many phosphorylation sites.
Significance
Previously, Phos-tag SDS-PAGE has been developed to identify a change in electrophoretic mobility of phosphoproteins. However, we had a problem in this technique; it was often difficult to recognize the mobility shift by Mn2+ Phos-tag when we used separately SDS-PAGE and Phos-tag SDS-PAGE. Such a problem was resolved by Phos-tag diagonal electrophoresis in which SDS-PAGE and Phos-tag SDS-PAGE patterns are provided on a single gel. This technique was useful to identify phosphorylation states of various proteins.
: Phos-tag diagonal electrophoresis, mass spectrometry, phosphoproteins, basic 7S globulin, proteasome
中文翻译:

Phos-tag对角电泳可精确检测Phos-tag SDS-PAGE中磷蛋白的迁移率变化
开发了Phos-tag对角电泳技术以精确鉴定Phos-tag SDS-PAGE中磷蛋白的电泳迁移率变化。以前,如果检测到单个蛋白条带,则无法确定在Phos-tag SDS-PAGE凝胶中Mn 2+ Phos-tag是否改变了蛋白质的迁移率,因为SDS-PAGE和Phos-tag SDS-PAGE是在不同的凝胶。此外,当检测到多个蛋白条带时,很难确定Mn 2+是否改变了迁移率最高的条带Phos标签。但是,这些问题通过Phos-tag对角电泳得以解决,其中在单个凝胶上提供SDS-PAGE和Phos-tag SDS-PAGE模式。使用此技术,我们鉴定了各种蛋白质(例如α-乳白蛋白,α-酪蛋白和β-酪蛋白,卵清蛋白,碱性7S球蛋白和26S蛋白酶体亚基)的磷酸化状态。在人类和酵母菌的26S蛋白酶体亚基的分析中,我们可以确认所有亚基都被磷酸化,并且发现尽管具有许多磷酸化位点,但每个亚基中具有不同磷酸化状态的主要蛋白质的数量却很少。
意义
以前,已经开发了磷酸标签SDS-PAGE来鉴定磷酸蛋白的电泳迁移率的变化。但是,我们在这项技术上存在问题;当我们分别使用SDS-PAGE和Phos-tag SDS-PAGE时,通常很难通过Mn 2+ Phos-tag识别迁移率变化。通过在单一凝胶上提供SDS-PAGE和Phos-tag SDS-PAGE模式的Phos-tag对角电泳解决了这个问题。该技术可用于鉴定各种蛋白质的磷酸化状态。
:磷酸标记对角电泳,质谱,磷蛋白,碱性7S球蛋白,蛋白酶体











































 京公网安备 11010802027423号
京公网安备 11010802027423号