JACC: Heart Failure ( IF 10.3 ) Pub Date : 2020-10-07 , DOI: 10.1016/j.jchf.2020.08.008 Justin A Ezekowitz 1 , Christopher M O'Connor 2 , Richard W Troughton 3 , Wendimagegn G Alemayehu 1 , Cynthia M Westerhout 1 , Adriaan A Voors 4 , Javed Butler 5 , Carolyn S P Lam 6 , Piotr Ponikowski 7 , Michele Emdin 8 , Mahesh J Patel 9 , Burkert Pieske 10 , Lothar Roessig 11 , Adrian F Hernandez 12 , Paul W Armstrong 1
|
Objectives
The purpose of this study was to examine the treatment effect of vericiguat in relation to N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels at randomization.
Background
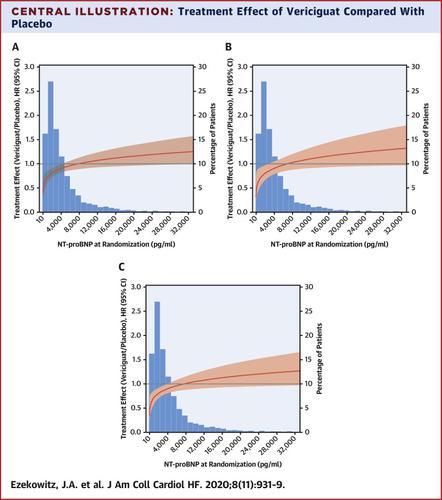
Vericiguat compared with placebo reduced the primary outcome of cardiovascular death (CVD) or heart failure hospitalization (HFH) in patients with HF with reduced ejection fraction (HFrEF) in the VICTORIA (A Study of Vericiguat in Participants With Heart Failure With Reduced Ejection Fraction) trial. Because an interaction existed between treatment and the primary outcome according to pre-specified quartiles of NT-proBNP at randomization, we examined this further.
Methods
This study evaluated the NT-proBNP relationship with the primary outcome in 4,805 of 5,050 patients as a risk-adjusted, log-transformed continuous variable. Hazard ratios (HRs) and 95% confidence intervals (CIs) are presented.
Results
Median NT-proBNP was 2,816 pg/ml (25th to 75th percentile: 1,556 to 5,314 pg/ml). The study treatment effect varied across the spectrum of NT-proBNP at randomization (with log2 transformation, p for interaction = 0.002). A significant association between treatment effects existed in patients with levels <4,000 pg/ml and remained evident up to 8,000 pg/ml. A 23% relative risk reduction occurred in the primary endpoint with NT-proBNP ≤4,000 pg/ml (HR: 0.77; 95% CI: 0.68 to 0.88). For NT-proBNP values ≤4,000 pg/ml (n = 3,100), the HR was 0.78 (95% CI: 0.67 to 0.90) for HFH and 0.75 (95% CI: 0.60 to 0.94) for CVD. For NT-proBNP ≤8,000 pg/ml (n = 4,133), the HR was 0.85 (95% CI: 0.76 to 0.95) for the primary outcome, 0.84 (95% CI: 0.75 to 0.95) for HFH, and 0.84 (95% CI: 0.71 to 0.99) for CVD. For NT-proBNP >8,000 pg/ml (n = 672), the HR was 1.16 (95% CI: 0.94 to 1.41) for the primary outcome.
Conclusions
A reduction in the primary composite endpoint and its CVD and HFH components was observed in patients on vericiguat compared with subjects on placebo with NT-proBNP levels up to 8,000 pg/ml. This provided new insight into the benefit observed in high-risk patients with worsening HFrEF. (A Study of Vericiguat in Participants With Heart Failure With Reduced Ejection Fraction [HFrEF] [MK-1242-001] [VICTORIA]; NCT02861534)
中文翻译:

N-Terminal Pro-B 型利钠肽和临床结果
目标
本研究的目的是检查 Vericiguat 在随机化时与 N 端 B 型利钠肽原 (NT-proBNP) 水平相关的治疗效果。
背景
Vericiguat 与安慰剂相比降低了 VICTORIA 中射血分数降低 (HFrEF) HF 患者的心血管死亡 (CVD) 或心力衰竭住院 (HFH) 的主要结局(一项 Vericiguat 在射血分数降低的心力衰竭参与者中的研究)审判。因为根据随机化时预先指定的 NT-proBNP 四分位数,治疗和主要结果之间存在相互作用,我们进一步研究了这一点。
方法
该研究评估了 NT-proBNP 与 5,050 名患者中的 4,805 名主要结果的关系,作为风险调整、对数转换的连续变量。提供了风险比 (HR) 和 95% 置信区间 (CI)。
结果
NT-proBNP 中位数为 2,816 pg/ml(第 25 至第 75 个百分位数:1,556 至 5,314 pg/ml)。研究治疗效果随 NT-proBNP 谱随机变化(log 2转换,交互作用的 p = 0.002)。治疗效果之间的显着关联存在于水平 <4,000 pg/ml 的患者中,并且在高达 8,000 pg/ml 的情况下仍然很明显。NT-proBNP ≤ 4,000 pg/ml 的主要终点相对风险降低了 23%(HR:0.77;95% CI:0.68 至 0.88)。NT-proBNP 值≤4,000 pg/ml (n = 3,100) 时,HFH 的 HR 为 0.78(95% CI:0.67 至 0.90),CVD 的 HR 为 0.75(95% CI:0.60 至 0.94)。对于 NT-proBNP ≤ 8,000 pg/ml (n = 4,133),主要结局的 HR 为 0.85(95% CI:0.76 至 0.95),HFH 的 HR 为 0.84(95% CI:0.75 至 0.95)和 0.84(95 % CI:0.71 至 0.99)对于 CVD。对于 NT-proBNP >8,000 pg/ml (n = 672),主要结局的 HR 为 1.16(95% CI:0.94 至 1.41)。
结论
与 NT-proBNP 水平高达 8,000 pg/ml 的安慰剂受试者相比,vericiguat 患者的主要复合终点及其 CVD 和 HFH 成分减少。这为在 HFrEF 恶化的高风险患者中观察到的益处提供了新的见解。(Vericiguat 对射血分数降低的心力衰竭患者的研究 [HFrEF] [MK-1242-001] [VICTORIA];NCT02861534)











































 京公网安备 11010802027423号
京公网安备 11010802027423号