当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Towards standard electrolytes for sodium-ion batteries: physical properties, ion solvation and ion-pairing in alkyl carbonate solvents
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-10-06 , DOI: 10.1039/d0cp03639k Damien Monti 1, 2, 3, 4, 5 , Erlendur Jónsson 1, 2, 3, 4, 6 , Andrea Boschin 1, 2, 3, 4 , M. Rosa Palacín 5, 7, 8, 9, 10 , Alexandre Ponrouch 5, 7, 8, 9, 10 , Patrik Johansson 1, 2, 3, 4, 9
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-10-06 , DOI: 10.1039/d0cp03639k Damien Monti 1, 2, 3, 4, 5 , Erlendur Jónsson 1, 2, 3, 4, 6 , Andrea Boschin 1, 2, 3, 4 , M. Rosa Palacín 5, 7, 8, 9, 10 , Alexandre Ponrouch 5, 7, 8, 9, 10 , Patrik Johansson 1, 2, 3, 4, 9
Affiliation
|
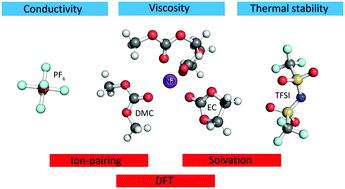
The currently emerging sodium-ion battery technology is in need of an optimized standard organic solvent electrolyte based on solid and directly comparable data. With this aim we have made a systematic study of “simple” electrolyte systems consisting of two sodium salts (NaTFSI and NaPF6) dissolved in three different alkyl carbonate solvents (EC, PC, DMC) within a wide range of salt concentrations and investigated: (i) their more macroscopic physico-chemical properties such as ionic conductivity, viscosity, thermal stability, and (ii) the molecular level properties such as ion-pairing and solvation. From this all electrolytes were found to have useful thermal operational windows and electrochemical stability windows, allowing for large scale energy storage technologies focused on load levelling or (to a less extent) electric vehicles, and ionic conductivities on par with analogous lithium-ion battery electrolytes, giving promise to also be power performant. Furthermore, at the molecular level the NaPF6-based electrolytes are more dissociated than the NaTFSI-based ones because of the higher ionic association strength of TFSI compared to PF6− while two different conformers of DMC participate in the Na+ first solvation shells – a Na+ affected conformational equilibrium and induced polarity of DMC. The non-negligible presence of DMC in the Na+ first solvation shells increases as a function of salt concentration. Overall, these results should both have a general impact on the design of more performant Na-conducting electrolytes and provide useful insight on the very details of the importance of DMC conformers in any cation solvation studies.
中文翻译:

面向钠离子电池的标准电解质:物理性质,离子溶剂化和碳酸烷基酯溶剂中的离子对
当前正在出现的钠离子电池技术需要基于固体和直接可比数据的优化标准有机溶剂电解质。为此,我们对由两种钠盐(NaTFSI和NaPF 6)在很宽的盐浓度范围内溶解在三种不同的碳酸烷基酯溶剂(EC,PC,DMC)中,并进行了以下研究:(i)其更宏观的物理化学性质,例如离子电导率,粘度,热稳定性,以及(ii)分子水平特性,例如离子对和溶剂化。由此发现所有电解质都具有有用的热操作窗口和电化学稳定性窗口,从而允许大规模能源存储技术专注于负载均衡或(在较小程度上)电动汽车,以及与类似锂离子电池电解质相当的离子电导率,也有望成为出色的表演者。此外,在分子水平上NaPF 6基电解质比基于NaTFSI的电解质更易解离,这是因为TFSI的离子缔合强度高于PF 6- ,而DMC的两个不同构象物参与了Na +第一溶剂化壳层-Na +影响的构象平衡和诱导的极性DMC。Na +第一溶剂化壳中DMC的不可忽略的存在随盐浓度的增加而增加。总体而言,这些结果都将对性能更高的含钠电解质的设计产生总体影响,并为在任何阳离子溶剂化研究中DMC构象异构体重要性的非常详细的细节提供有用的见解。
更新日期:2020-10-16
中文翻译:

面向钠离子电池的标准电解质:物理性质,离子溶剂化和碳酸烷基酯溶剂中的离子对
当前正在出现的钠离子电池技术需要基于固体和直接可比数据的优化标准有机溶剂电解质。为此,我们对由两种钠盐(NaTFSI和NaPF 6)在很宽的盐浓度范围内溶解在三种不同的碳酸烷基酯溶剂(EC,PC,DMC)中,并进行了以下研究:(i)其更宏观的物理化学性质,例如离子电导率,粘度,热稳定性,以及(ii)分子水平特性,例如离子对和溶剂化。由此发现所有电解质都具有有用的热操作窗口和电化学稳定性窗口,从而允许大规模能源存储技术专注于负载均衡或(在较小程度上)电动汽车,以及与类似锂离子电池电解质相当的离子电导率,也有望成为出色的表演者。此外,在分子水平上NaPF 6基电解质比基于NaTFSI的电解质更易解离,这是因为TFSI的离子缔合强度高于PF 6- ,而DMC的两个不同构象物参与了Na +第一溶剂化壳层-Na +影响的构象平衡和诱导的极性DMC。Na +第一溶剂化壳中DMC的不可忽略的存在随盐浓度的增加而增加。总体而言,这些结果都将对性能更高的含钠电解质的设计产生总体影响,并为在任何阳离子溶剂化研究中DMC构象异构体重要性的非常详细的细节提供有用的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号