当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Target-driven supramolecular self-assembly for selective amyloid-β photooxygenation against Alzheimer's disease
Chemical Science ( IF 7.6 ) Pub Date : 2020-10-06 , DOI: 10.1039/d0sc04984k Zhenqi Liu 1, 2 , Mengmeng Ma 1, 2 , Dongqin Yu 1, 2 , Jinsong Ren 1, 2 , Xiaogang Qu 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2020-10-06 , DOI: 10.1039/d0sc04984k Zhenqi Liu 1, 2 , Mengmeng Ma 1, 2 , Dongqin Yu 1, 2 , Jinsong Ren 1, 2 , Xiaogang Qu 1, 2
Affiliation
|
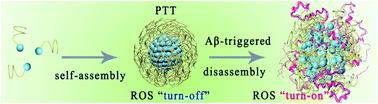
Photo-oxygenation of β-amyloid (Aβ) has been considered an efficient way to inhibit Aβ aggregation in Alzheimer's disease (AD). However, current photosensitizers cannot simultaneously achieve enhanced blood–brain barrier (BBB) permeability and selective photooxygenation of Aβ, leading to poor therapeutic efficacy, severe off-target toxicity, and substandard bioavailability. Herein, an Aβ target-driven supramolecular self-assembly (PKNPs) with enhanced BBB penetrability and switchable photoactivity is designed and demonstrated to be effective in preventing Aβ aggregation in vivo. PKNPs are prepared by the self-assembly of the Aβ-targeting peptide KLVFF and an FDA-approved porphyrin derivative (5-(4-carboxyphenyl)-10,15,20-triphenylporphyrin). Due to the photothermal effect of PKNPs, the BBB permeability of PKNPs under irradiation is 8.5-fold higher than that of porphyrin alone. Moreover, upon selective interaction with Aβ, PKNPs undergo morphological change from the spherical to the amorphous form, resulting in a smart transformation from photothermal activity to photodynamic activity. Consequently, the disassembled PKNPs can selectively oxygenate Aβ without affecting off-target proteins (insulin, bovine serum albumin, and human serum albumin). The well-designed PKNPs exhibit not only improved BBB permeability but also highly selective Aβ photooxygenation. Furthermore, in vivo experiments demonstrate that PKNPs can alleviate Aβ-induced neurotoxicity and prolong the life span of the commonly used AD transgenic Caenorhabditis elegans CL2006. Our work may open a new path for using supramolecular self-assemblies as switchable phototheranostics for the selective and effective prevention of Aβ aggregation and related neurotoxicity in AD.
中文翻译:

靶向驱动的超分子自组装用于选择性淀粉样蛋白-β光氧合对抗阿尔茨海默病
β-淀粉样蛋白 (Aβ) 的光氧化被认为是抑制阿尔茨海默病 (AD) 中 Aβ 聚集的有效方法。然而,目前的光敏剂无法同时实现增强血脑屏障(BBB)通透性和Aβ的选择性光氧合,导致治疗效果差、脱靶毒性严重和生物利用度不合格。在此,设计了一种具有增强 BBB 穿透性和可切换光活性的 Aβ 靶驱动超分子自组装(PKNP),并证明可有效防止体内Aβ 聚集。 PKNP 通过 Aβ 靶向肽 KLVFF 和 FDA 批准的卟啉衍生物(5-(4-羧基苯基)-10,15,20-三苯基卟啉)的自组装来制备。由于PKNPs的光热效应,辐照下PKNPs的BBB渗透性比单独的卟啉高8.5倍。此外,在与 Aβ 选择性相互作用后,PKNP 会发生从球形到无定形的形态变化,从而实现从光热活性到光动力活性的智能转变。因此,分解的 PKNP 可以选择性地氧化 Aβ,而不影响脱靶蛋白(胰岛素、牛血清白蛋白和人血清白蛋白)。精心设计的 PKNP 不仅表现出改善的 BBB 通透性,而且具有高度选择性的 Aβ 光氧合作用。此外,体内实验表明,PKNPs 可以减轻 Aβ 诱导的神经毒性并延长常用的 AD 转基因秀丽隐杆线虫CL2006 的寿命。 我们的工作可能为使用超分子自组装体作为可切换的光治疗药物来选择性和有效地预防 AD 中 Aβ 聚集和相关神经毒性开辟了一条新途径。
更新日期:2020-10-13
中文翻译:

靶向驱动的超分子自组装用于选择性淀粉样蛋白-β光氧合对抗阿尔茨海默病
β-淀粉样蛋白 (Aβ) 的光氧化被认为是抑制阿尔茨海默病 (AD) 中 Aβ 聚集的有效方法。然而,目前的光敏剂无法同时实现增强血脑屏障(BBB)通透性和Aβ的选择性光氧合,导致治疗效果差、脱靶毒性严重和生物利用度不合格。在此,设计了一种具有增强 BBB 穿透性和可切换光活性的 Aβ 靶驱动超分子自组装(PKNP),并证明可有效防止体内Aβ 聚集。 PKNP 通过 Aβ 靶向肽 KLVFF 和 FDA 批准的卟啉衍生物(5-(4-羧基苯基)-10,15,20-三苯基卟啉)的自组装来制备。由于PKNPs的光热效应,辐照下PKNPs的BBB渗透性比单独的卟啉高8.5倍。此外,在与 Aβ 选择性相互作用后,PKNP 会发生从球形到无定形的形态变化,从而实现从光热活性到光动力活性的智能转变。因此,分解的 PKNP 可以选择性地氧化 Aβ,而不影响脱靶蛋白(胰岛素、牛血清白蛋白和人血清白蛋白)。精心设计的 PKNP 不仅表现出改善的 BBB 通透性,而且具有高度选择性的 Aβ 光氧合作用。此外,体内实验表明,PKNPs 可以减轻 Aβ 诱导的神经毒性并延长常用的 AD 转基因秀丽隐杆线虫CL2006 的寿命。 我们的工作可能为使用超分子自组装体作为可切换的光治疗药物来选择性和有效地预防 AD 中 Aβ 聚集和相关神经毒性开辟了一条新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号