当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 reduction and ethane dehydrogenation on transition metal catalysts: mechanistic insights, reactivity trends and rational design of bimetallic alloys
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-10-05 , DOI: 10.1039/d0cy01290d Fatima Jalid 1, 2, 3, 4, 5 , Tuhin Suvra Khan 5, 6, 7, 8 , M. Ali Haider 1, 2, 3, 4, 5
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2020-10-05 , DOI: 10.1039/d0cy01290d Fatima Jalid 1, 2, 3, 4, 5 , Tuhin Suvra Khan 5, 6, 7, 8 , M. Ali Haider 1, 2, 3, 4, 5
Affiliation
|
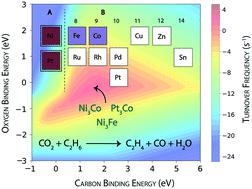
Reactivity trends of transition metal catalysts are studied for the ethane dehydrogenation reaction using CO2 as a mild oxidant. An ab initio microkinetic model (MKM) is constructed to gain insights about the dominant route for CO2 reduction and simultaneous ethylene formation over the terrace (111) and step (211) surfaces of the catalysts. At the terrace sites, Rh and Pt are observed to show high ethane consumption with maximum turnovers to produce ethylene. For CO2 consumption, Rh, Ru, Ni and Co are calculated to exhibit significant activity (TOF ∼1 s−1). CO2 on the (111) surface is predominantly reduced through the reverse water gas shift (RWGS) reaction, since the production rates of H2O and CO are comparable to the consumption rates of CO2. At the step sites, the hydrogenolysis reaction is more pronounced leading to coke formation. Hydrogenolysis at the step surface also led to significant activity for the reforming reaction. Over the (211) surface, the direct dehydrogenation of ethane to produce ethylene is observed to be predominant. For oxygen assisted ethane dehydrogenation, Co, Ru, Ni and Rh are calculated to show appreciable activity (>1 s−1). The same four metals also show significant CO2 consumption at the step surface. The MKM is further utilised to design bimetallic alloys of Ni and Pt to achieve greater CO2 consumption activity and reduced coke formation with significant activity for the dehydrogenation reaction. While most of the alloys undergo reforming and RWGS reactions, three potential bimetallic combinations (NiFe, NiCo and PtCo) are selected, exhibiting appreciable activity for CO2 assisted dehydrogenation of ethane with some reduction in coke formation, compared to their monometallic counterparts.
中文翻译:

过渡金属催化剂上的CO2还原和乙烷脱氢:双金属合金的力学见解,反应性趋势和合理设计
研究了过渡金属催化剂在以CO 2为弱氧化剂的乙烷脱氢反应中的反应趋势。一个从头microkinetic模型(MKM)被构造成获得关于对CO的主导路径见解2还原和同时形成乙烯在露台(111)和步骤(211)催化剂的表面。在梯田处,观察到Rh和Pt的乙烷消耗量很高,而生产乙烯的周转量最大。对于消耗的CO 2,计算得出Rh,Ru,Ni和Co具有明显的活性(TOF〜1 s -1)。一氧化碳2(111)表面上的H 2 O和CO的产生速率与CO 2的消耗速率相当,因此主要是通过逆水煤气变换(RWGS)反应来减少。在台阶处,氢解反应更加明显,导致形成焦炭。在台阶表面的氢解也导致重整反应的显着活性。在(211)表面上,主要观察到乙烷直接脱氢生成乙烯。对于氧辅助的乙烷脱氢,计算出Co,Ru,Ni和Rh以显示出明显的活性(> 1 s -1)。同样的四种金属也显示出显着的CO 2在踏板表面的消耗。MKM还被用于设计Ni和Pt的双金属合金,以实现更大的CO 2消耗活性和减少的焦炭形成,并具有显着的脱氢反应活性。尽管大多数合金都经过重整和RWGS反应,但选择了三种潜在的双金属组合(NiFe,NiCo和PtCo),与单金属对应物相比,它们对CO 2辅助的乙烷脱氢表现出可观的活性,并减少了焦炭形成。
更新日期:2020-11-03
中文翻译:

过渡金属催化剂上的CO2还原和乙烷脱氢:双金属合金的力学见解,反应性趋势和合理设计
研究了过渡金属催化剂在以CO 2为弱氧化剂的乙烷脱氢反应中的反应趋势。一个从头microkinetic模型(MKM)被构造成获得关于对CO的主导路径见解2还原和同时形成乙烯在露台(111)和步骤(211)催化剂的表面。在梯田处,观察到Rh和Pt的乙烷消耗量很高,而生产乙烯的周转量最大。对于消耗的CO 2,计算得出Rh,Ru,Ni和Co具有明显的活性(TOF〜1 s -1)。一氧化碳2(111)表面上的H 2 O和CO的产生速率与CO 2的消耗速率相当,因此主要是通过逆水煤气变换(RWGS)反应来减少。在台阶处,氢解反应更加明显,导致形成焦炭。在台阶表面的氢解也导致重整反应的显着活性。在(211)表面上,主要观察到乙烷直接脱氢生成乙烯。对于氧辅助的乙烷脱氢,计算出Co,Ru,Ni和Rh以显示出明显的活性(> 1 s -1)。同样的四种金属也显示出显着的CO 2在踏板表面的消耗。MKM还被用于设计Ni和Pt的双金属合金,以实现更大的CO 2消耗活性和减少的焦炭形成,并具有显着的脱氢反应活性。尽管大多数合金都经过重整和RWGS反应,但选择了三种潜在的双金属组合(NiFe,NiCo和PtCo),与单金属对应物相比,它们对CO 2辅助的乙烷脱氢表现出可观的活性,并减少了焦炭形成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号