当前位置:
X-MOL 学术
›
ACS Pharmacol. Transl. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Heterodimeric Insecticidal Peptide Provides New Insights into the Molecular and Functional Diversity of Ant Venoms
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-10-06 , DOI: 10.1021/acsptsci.0c00119 Axel Touchard 1 , Helen C Mendel 2 , Isabelle Boulogne 3 , Volker Herzig 2, 4 , Nayara Braga Emidio 2 , Glenn F King 2 , Mathilde Triquigneaux 5 , Lucie Jaquillard 5 , Rémy Beroud 5 , Michel De Waard 5, 6, 7 , Olivier Delalande 8 , Alain Dejean 1, 9 , Markus Muttenthaler 2, 10 , Christophe Duplais 1
ACS Pharmacology & Translational Science ( IF 4.9 ) Pub Date : 2020-10-06 , DOI: 10.1021/acsptsci.0c00119 Axel Touchard 1 , Helen C Mendel 2 , Isabelle Boulogne 3 , Volker Herzig 2, 4 , Nayara Braga Emidio 2 , Glenn F King 2 , Mathilde Triquigneaux 5 , Lucie Jaquillard 5 , Rémy Beroud 5 , Michel De Waard 5, 6, 7 , Olivier Delalande 8 , Alain Dejean 1, 9 , Markus Muttenthaler 2, 10 , Christophe Duplais 1
Affiliation
|
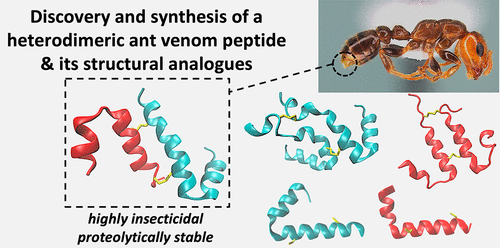
Ants use venom for predation, defense, and communication; however, the molecular diversity, function, and potential applications of ant venom remains understudied compared to other venomous lineages such as arachnids, snakes and cone snails. In this work, we used a multidisciplinary approach that encompassed field work, proteomics, sequencing, chemical synthesis, structural analysis, molecular modeling, stability studies, and in vitro and in vivo bioassays to investigate the molecular diversity of the venom of the Amazonian Pseudomyrmex penetrator ants. We isolated a potent insecticidal heterodimeric peptide Δ-pseudomyrmecitoxin-Pp1a (Δ-PSDTX-Pp1a) composed of a 27-residue long A-chain and a 33-residue long B-chain cross-linked by two disulfide bonds in an antiparallel orientation. We chemically synthesized Δ-PSDTX-Pp1a, its corresponding parallel AA and BB homodimers, and its monomeric chains and demonstrated that Δ-PSDTX-Pp1a had the most potent insecticidal effects in blowfly assays (LD50 = 3 nmol/g). Molecular modeling and circular dichroism studies revealed strong α-helical features, indicating its cytotoxic effects could derive from cell membrane pore formation or disruption. The native heterodimer was substantially more stable against proteolytic degradation (t1/2 = 13 h) than its homodimers or monomers (t1/2 < 20 min), indicating an evolutionary advantage of the more complex structure. The proteomic analysis of Pseudomyrmex penetrator venom and in-depth characterization of Δ-PSDTX-Pp1a provide novel insights in the structural complexity of ant venom and further exemplifies how nature exploits disulfide-bond formation and dimerization to gain an evolutionary advantage via improved stability, a concept that is highly relevant for the design and development of peptide therapeutics, molecular probes, and bioinsecticides.
中文翻译:

异二聚体杀虫肽为蚂蚁毒液的分子和功能多样性提供了新的见解
蚂蚁使用毒液进行捕食、防御和交流;然而,与蜘蛛、蛇和锥蜗牛等其他有毒谱系相比,蚂蚁毒液的分子多样性、功能和潜在应用仍未得到充分研究。在这项工作中,我们使用了包括实地工作、蛋白质组学、测序、化学合成、结构分析、分子建模、稳定性研究以及体外和体内生物测定在内的多学科方法来研究亚马逊假单胞菌穿透剂毒液的分子多样性蚂蚁。我们分离了一种有效的杀虫异二聚体肽 Δ-pseudomyrmecitoxin-Pp1a (Δ-PSDTX-Pp1a),由 27 个残基的长 A 链和 33 个残基的长 B 链以反平行方向通过两个二硫键交联而成。我们化学合成了 Δ-PSDTX-Pp1a、其相应的平行 AA 和 BB 同源二聚体及其单体链,并证明 Δ-PSDTX-Pp1a 在苍蝇试验中具有最有效的杀虫作用 (LD 50 = 3 nmol/g)。分子建模和圆二色性研究揭示了强烈的α-螺旋特征,表明其细胞毒性作用可能来自细胞膜孔的形成或破坏。与同源二聚体或单体相比,天然异源二聚体对蛋白水解降解 ( t 1/2 = 13 h) 的稳定性要高得多。t 1/2 < 20 分钟),表明更复杂结构的进化优势。Pseudomyrmex 穿透毒液的蛋白质组学分析和 Δ-PSDTX-Pp1a 的深入表征为蚂蚁毒液的结构复杂性提供了新的见解,并进一步举例说明了自然界如何利用二硫键形成和二聚化来通过提高稳定性获得进化优势,这一概念与肽疗法、分子探针和生物杀虫剂的设计和开发高度相关。
更新日期:2020-12-12
中文翻译:

异二聚体杀虫肽为蚂蚁毒液的分子和功能多样性提供了新的见解
蚂蚁使用毒液进行捕食、防御和交流;然而,与蜘蛛、蛇和锥蜗牛等其他有毒谱系相比,蚂蚁毒液的分子多样性、功能和潜在应用仍未得到充分研究。在这项工作中,我们使用了包括实地工作、蛋白质组学、测序、化学合成、结构分析、分子建模、稳定性研究以及体外和体内生物测定在内的多学科方法来研究亚马逊假单胞菌穿透剂毒液的分子多样性蚂蚁。我们分离了一种有效的杀虫异二聚体肽 Δ-pseudomyrmecitoxin-Pp1a (Δ-PSDTX-Pp1a),由 27 个残基的长 A 链和 33 个残基的长 B 链以反平行方向通过两个二硫键交联而成。我们化学合成了 Δ-PSDTX-Pp1a、其相应的平行 AA 和 BB 同源二聚体及其单体链,并证明 Δ-PSDTX-Pp1a 在苍蝇试验中具有最有效的杀虫作用 (LD 50 = 3 nmol/g)。分子建模和圆二色性研究揭示了强烈的α-螺旋特征,表明其细胞毒性作用可能来自细胞膜孔的形成或破坏。与同源二聚体或单体相比,天然异源二聚体对蛋白水解降解 ( t 1/2 = 13 h) 的稳定性要高得多。t 1/2 < 20 分钟),表明更复杂结构的进化优势。Pseudomyrmex 穿透毒液的蛋白质组学分析和 Δ-PSDTX-Pp1a 的深入表征为蚂蚁毒液的结构复杂性提供了新的见解,并进一步举例说明了自然界如何利用二硫键形成和二聚化来通过提高稳定性获得进化优势,这一概念与肽疗法、分子探针和生物杀虫剂的设计和开发高度相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号