Science Bulletin ( IF 18.8 ) Pub Date : 2020-10-06 , DOI: 10.1016/j.scib.2020.10.001 Binzhi Zhao 1 , Yuqiang Li 1 , Haoyang Li 1 , Md Belal 1 , Lei Zhu 2 , Guoyin Yin 1
|
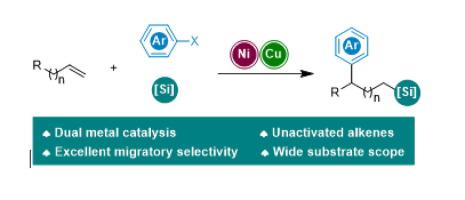
Synthesis of organosilanes from alkenes is a very important topic owing to their wide applications. A Ni/Cu dual metal-catalyzed arylsilylation of terminal alkenes, featuring migratory selectivity, has been developed. A wide diversity of aliphatic silanes have been prepared from terminal alkenes, aryl halides and Suginome’s reagent. This protocol is highlighted by excellent regioselectivity, mild reaction conditions and good functional group tolerance. In addition to benzylic positions, carbon–carbon bonds can also be constructed at allylic positions. Preliminary mechanistic studies suggest that the copper cocatalyst promotes the transmetalation of Suginome’s reagent, and the addition of a PyrOx ligand inhibits the formation of side-products from the carbon-Heck pathway. Moreover, studies toward the nature of the PyrOx ligand revealed that the steric hindrance of the oxazoline moiety greatly affects the chain–walking process, but not the arylation step.
中文翻译:

协同Ni/Cu催化末端烯烃的迁移芳基硅烷化
由于烯烃的广泛应用,由烯烃合成有机硅烷是一个非常重要的课题。已开发出具有迁移选择性的 Ni/Cu 双金属催化的末端烯烃芳基硅烷化反应。从末端烯烃、芳基卤化物和 Suginome 试剂制备了多种脂肪族硅烷。该方案具有出色的区域选择性、温和的反应条件和良好的官能团耐受性。除了苄基位置,碳-碳键也可以在烯丙基位置构建。初步的机理研究表明,铜助催化剂促进了 Suginome 试剂的金属转移,而 PyrOx 配体的添加抑制了碳-Heck 途径副产物的形成。而且,











































 京公网安备 11010802027423号
京公网安备 11010802027423号