Journal of Inorganic Biochemistry ( IF 3.8 ) Pub Date : 2020-10-06 , DOI: 10.1016/j.jinorgbio.2020.111277 Bianca Boni Dias 1 , Fabiana Gomes da Silva Dantas 2 , Fernanda Galvão 1 , Wellinton Jhon Cupozak-Pinheiro 3 , Heberton Wender 4 , Lucas Pizzuti 5 , Persiely Pires Rosa 5 , Kátia Veronica Tenório 5 , Claudia Cristina Gatto 6 , Melyssa Negri 7 , Gleison Antônio Casagrande 5 , Kelly Mari Pires de Oliveira 8
|
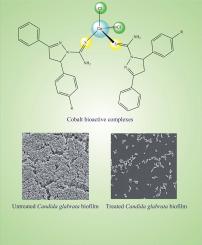
Candida spp. cause invasive fungal infections. One species, Candida glabrata, may present intrinsic resistance to conventional antifungal agents, thereby increasing mortality rates in hospitalized patients. In this context, metal complexes present an alternative for the development of new antifungal drugs owing to their biological and pharmacological activities demonstrated in studies in the last decades. Accordingly, in this study we have synthesized and characterized two new Co(II) complexes with thiocarbamoyl-pyrazoline ligands to assess their antimicrobial, mutagenic, and cytotoxic potential. For antimicrobial activity, the broth microdilution method was performed against ATCC strains of Candida spp. and fluconazole dose-dependent isolates of C. glabrata obtained from urine samples. The Ames test was used to assess mutagenic potential. The reduction method of the MTS reagent (3 [4,5-dimethylthiazol-2-yl]-5-[3-carboxymethoxyphenyl]-2-[4-sulfophenyl]-2H-tetrazolium) was performed with HeLa, SiHa, and Vero cells to determine cytotoxicity. Both complexes exhibited fungistatic and fungicidal activity for the yeasts used in the study, demonstrating greater potential for C. glabrata ATCC 2001 and the C. glabrata CG66 isolate with a Minimum Inhibitory Concentration MIC from 3.90 to 7.81 μg mL−1 and fungicidal action from 7.81 to 15.62 μg mL−1. The complexes inhibited and degraded biofilms by up to 90% and did not present mutagenic and cytotoxic potential at the concentrations evaluated for MIC. Thus, the complexes examined herein suggest promising alternatives for the development of new antifungal drugs.
中文翻译:

新型钴(II)与硫代氨基甲酰基-吡唑啉配体作为有希望的抗真菌剂的配合物的合成,结构表征和前景
念珠菌属 引起侵入性真菌感染。一种物种,光滑念珠菌可能表现出对常规抗真菌剂的内在抗性,从而增加住院患者的死亡率。在这种情况下,金属络合物由于最近几十年的研究表明具有生物和药理活性,因此成为开发新的抗真菌药物的替代方法。因此,在这项研究中,我们合成并表征了两种新的具有硫代氨基甲酰基吡唑啉配体的Co(II)配合物,以评估其抗微生物,诱变和细胞毒性的潜力。对于抗菌活性,对念珠菌属的ATCC菌株进行了肉汤微稀释法。的和氟康唑剂量依赖性分离光滑念珠菌从尿液样本中获得。使用Ames测试评估诱变潜力。用HeLa,SiHa和Vero进行MTS试剂(3 [4,5-二甲基噻唑-2-基] -5- [3-羧基甲氧基苯基] -2- [4-磺基苯基] -2H-四唑鎓)的还原方法细胞以确定细胞毒性。两种复合表现出抑制真菌和杀真菌活性在研究中使用的酵母,展示出更大的潜力为光滑念珠菌2001 ATCC和光滑念珠菌CG66分离物用最小抑制浓度MIC从3.90到7.81微克毫升-1和从7.81杀真菌作用至15.62μgmL -1。该复合物抑制和降解生物膜的可能性高达90%,并且在MIC评估浓度下不表现出诱变和细胞毒性潜力。因此,本文检查的复合物为开发新的抗真菌药物提出了有希望的替代方法。










































 京公网安备 11010802027423号
京公网安备 11010802027423号