当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Compositional effect on water adsorption on metal halide perovskites
Applied Surface Science ( IF 6.7 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148058 Qihua Li , Zehua Chen , Ionut Tranca , Silvia Gaastra-Nedea , David Smeulders , Shuxia Tao
Applied Surface Science ( IF 6.7 ) Pub Date : 2021-02-01 , DOI: 10.1016/j.apsusc.2020.148058 Qihua Li , Zehua Chen , Ionut Tranca , Silvia Gaastra-Nedea , David Smeulders , Shuxia Tao
|
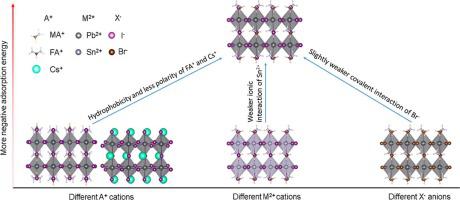
Abstract The moisture-induced instability of metal halide perovskites is one of the major challenges for perovskite devices. Although compositional engineering has been widely employed to improve the overall stability of perovskites, its effect on the moisture-induced instability received little attention. Here, we systematically study the interaction of water with the surfaces of primary perovskites, AMX3 (A+ = MA+, FA+, Cs+; M2+ = Pb2+, Sn2+; X− = I−, Br−), by using Density Functional Theory (DFT) calculations and comprehensive chemical bonding analysis. We reveal that the hydrophilic group NH3+ of MA+ cation may be the cause for instability issues. We find that the adsorption of water on FAPbI3 and CsPbI3 are much weaker than on MAPbI3 due to the less polarity of FA+ and Cs+. When exchanging M2+ cations, water adsorption on MASnI3 is also less energetically favorable than on MAPbI3 because of the weaker ionic interaction of H2O-MASnI3. When exchanging X− anion, water adsorption on MAPbBr3 is slightly weaker than on MAPbI3 due to the slightly weaker covalent interaction of H2O-MAPbBr3. Our results present a comprehensive understanding of the compositional effect on the interactions of water with perovskites and provide rational design strategies to improve their stability against moisture via compositional engineering.
中文翻译:

金属卤化物钙钛矿吸水的组成效应
摘要 金属卤化物钙钛矿的水分引起的不稳定性是钙钛矿器件面临的主要挑战之一。尽管成分工程已被广泛用于提高钙钛矿的整体稳定性,但其对水分引起的不稳定性的影响却很少受到关注。在这里,我们系统地研究了水与初级钙钛矿 AMX3 表面的相互作用(A+ = MA+、FA+、Cs+;M2+ = Pb2+、Sn2+;X− = I−、Br−),通过使用密度泛函理论 (DFT)计算和综合化学键分析。我们发现 MA+ 阳离子的亲水基团 NH3+ 可能是不稳定问题的原因。我们发现水在 FAPbI3 和 CsPbI3 上的吸附比在 MAPbI3 上弱得多,因为 FA+ 和 Cs+ 的极性较小。当交换 M2+ 阳离子时,由于 H2O-MASnI3 的离子相互作用较弱,因此 MASnI3 上的水吸附在能量上也不如 MAPbI3。当交换 X− 阴离子时,由于 H2O-MAPbBr3 的共价相互作用稍弱,MAPbBr3 上的水吸附略弱于 MAPbI3。我们的研究结果提供了对水与钙钛矿相互作用的成分影响的全面理解,并提供了合理的设计策略,以通过成分工程提高其对水分的稳定性。
更新日期:2021-02-01
中文翻译:

金属卤化物钙钛矿吸水的组成效应
摘要 金属卤化物钙钛矿的水分引起的不稳定性是钙钛矿器件面临的主要挑战之一。尽管成分工程已被广泛用于提高钙钛矿的整体稳定性,但其对水分引起的不稳定性的影响却很少受到关注。在这里,我们系统地研究了水与初级钙钛矿 AMX3 表面的相互作用(A+ = MA+、FA+、Cs+;M2+ = Pb2+、Sn2+;X− = I−、Br−),通过使用密度泛函理论 (DFT)计算和综合化学键分析。我们发现 MA+ 阳离子的亲水基团 NH3+ 可能是不稳定问题的原因。我们发现水在 FAPbI3 和 CsPbI3 上的吸附比在 MAPbI3 上弱得多,因为 FA+ 和 Cs+ 的极性较小。当交换 M2+ 阳离子时,由于 H2O-MASnI3 的离子相互作用较弱,因此 MASnI3 上的水吸附在能量上也不如 MAPbI3。当交换 X− 阴离子时,由于 H2O-MAPbBr3 的共价相互作用稍弱,MAPbBr3 上的水吸附略弱于 MAPbI3。我们的研究结果提供了对水与钙钛矿相互作用的成分影响的全面理解,并提供了合理的设计策略,以通过成分工程提高其对水分的稳定性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号