当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cell‐type‐specific hypertranslocation of effectors by the Pseudomonas aeruginosa type III secretion system
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-10-04 , DOI: 10.1111/mmi.14617 Erin I Armentrout 1 , Emma C Kundracik 1 , Arne Rietsch 1
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-10-04 , DOI: 10.1111/mmi.14617 Erin I Armentrout 1 , Emma C Kundracik 1 , Arne Rietsch 1
Affiliation
|
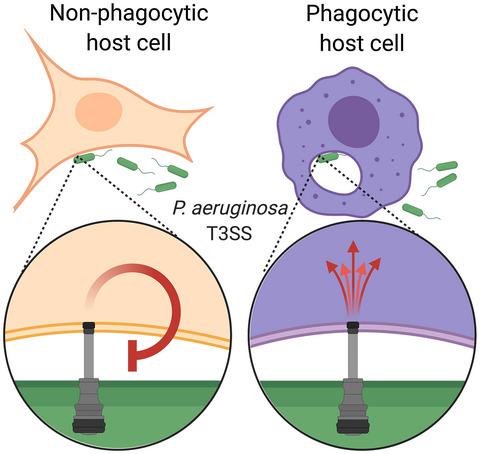
Many Gram‐negative pathogens use a type III secretion system (T3SS) to promote disease by injecting effector proteins into host cells. Common to many T3SSs is that injection of effector proteins is feedback inhibited. The mechanism of feedback inhibition and its role in pathogenesis are unclear. In the case of P. aeruginosa, the effector protein ExoS is central to limiting effector injection. ExoS is bifunctional, with an amino‐terminal RhoGAP and a carboxy‐terminal ADP‐ribosyltransferase domain. We demonstrate that both domains are required to fully feedback inhibit effector injection. The RhoGAP‐, but not the ADP‐ribosyltransferase domain of the related effector protein ExoT also participates. Feedback inhibition does not involve translocator insertion nor pore‐formation. Instead, feedback inhibition is due, in part, to a loss of the activating trigger for effector injection, and likely also decreased translocon stability. Surprisingly, feedback inhibition is abrogated in phagocytic cells. The lack of feedback inhibition in these cells requires phagocytic uptake of the bacteria, but cannot be explained through acidification of the phagosome or calcium limitation. Given that phagocytes are crucial for controlling P. aeruginosa infections, our data suggest that feedback inhibition allows P. aeruginosa to direct its effector arsenal against the cell types most damaging to its survival.
中文翻译:

铜绿假单胞菌 III 型分泌系统对效应子的细胞类型特异性超易位
许多革兰氏阴性病原体使用 III 型分泌系统 (T3SS) 通过将效应蛋白注入宿主细胞来促进疾病。许多 T3SS 的共同点是效应蛋白的注射受到反馈抑制。反馈抑制的机制及其在发病机制中的作用尚不清楚。在绿脓杆菌的情况下,效应蛋白 ExoS 是限制效应注射的核心。ExoS 是双功能的,具有氨基末端 RhoGAP 和羧基末端 ADP-核糖基转移酶结构域。我们证明这两个域都需要完全反馈抑制效应注入。相关效应蛋白 ExoT 的 RhoGAP-,但不是 ADP-核糖基转移酶结构域也参与其中。反馈抑制不涉及转运蛋白插入或孔形成。相反,反馈抑制部分是由于失去了效应器注射的激活触发器,并且可能还降低了易位子的稳定性。令人惊讶的是,吞噬细胞中的反馈抑制被消除了。这些细胞中缺乏反馈抑制需要细菌的吞噬吸收,但不能通过吞噬体的酸化或钙限制来解释。铜绿假单胞菌感染,我们的数据表明,反馈抑制允许铜绿假单胞菌将其效应器库引导至对其生存最有害的细胞类型。
更新日期:2020-10-04
中文翻译:

铜绿假单胞菌 III 型分泌系统对效应子的细胞类型特异性超易位
许多革兰氏阴性病原体使用 III 型分泌系统 (T3SS) 通过将效应蛋白注入宿主细胞来促进疾病。许多 T3SS 的共同点是效应蛋白的注射受到反馈抑制。反馈抑制的机制及其在发病机制中的作用尚不清楚。在绿脓杆菌的情况下,效应蛋白 ExoS 是限制效应注射的核心。ExoS 是双功能的,具有氨基末端 RhoGAP 和羧基末端 ADP-核糖基转移酶结构域。我们证明这两个域都需要完全反馈抑制效应注入。相关效应蛋白 ExoT 的 RhoGAP-,但不是 ADP-核糖基转移酶结构域也参与其中。反馈抑制不涉及转运蛋白插入或孔形成。相反,反馈抑制部分是由于失去了效应器注射的激活触发器,并且可能还降低了易位子的稳定性。令人惊讶的是,吞噬细胞中的反馈抑制被消除了。这些细胞中缺乏反馈抑制需要细菌的吞噬吸收,但不能通过吞噬体的酸化或钙限制来解释。铜绿假单胞菌感染,我们的数据表明,反馈抑制允许铜绿假单胞菌将其效应器库引导至对其生存最有害的细胞类型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号