Molecular Cell ( IF 16.0 ) Pub Date : 2020-10-05 , DOI: 10.1016/j.molcel.2020.09.017 Franziska Walser 1 , Monique P C Mulder 2 , Benoît Bragantini 3 , Sibylle Burger 1 , Tatiana Gubser 1 , Marco Gatti 4 , Maria Victoria Botuyan 3 , Alessandra Villa 5 , Matthias Altmeyer 4 , Dario Neri 5 , Huib Ovaa 2 , Georges Mer 3 , Lorenza Penengo 1
|
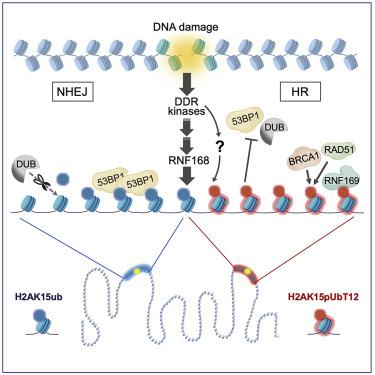
The ubiquitin system regulates the DNA damage response (DDR) by modifying histone H2A at Lys15 (H2AK15ub) and triggering downstream signaling events. Here, we find that phosphorylation of ubiquitin at Thr12 (pUbT12) controls the DDR by inhibiting the function of 53BP1, a key factor for DNA double-strand break repair by non-homologous end joining (NHEJ). Detectable as a chromatin modification on H2AK15ub, pUbT12 accumulates in nuclear foci and is increased upon DNA damage. Mutating Thr12 prevents the removal of ubiquitin from H2AK15ub by USP51 deubiquitinating enzyme, leading to a pronounced accumulation of ubiquitinated chromatin. Chromatin modified by pUbT12 is inaccessible to 53BP1 but permissive to the homologous recombination (HR) proteins RNF169, RAD51, and the BRCA1/BARD1 complex. Phosphorylation of ubiquitin at Thr12 in the chromatin context is a new histone mark, H2AK15pUbT12, that regulates the DDR by hampering the activity of 53BP1 at damaged chromosomes.
中文翻译:

Thr12 处的泛素磷酸化调节 DNA 损伤反应
泛素系统通过修饰 Lys15 (H2AK15ub) 的组蛋白 H2A 并触发下游信号事件来调节 DNA 损伤反应 (DDR)。在这里,我们发现 Thr12 (pUbT12) 泛素的磷酸化通过抑制 53BP1 的功能来控制 DDR,53BP1 是通过非同源末端连接 (NHEJ) 修复 DNA 双链断裂的关键因素。可检测到 H2AK15ub 上的染色质修饰,pUbT12 在核病灶中积累并且在 DNA 损伤时增加。突变的 Thr12 阻止了 USP51 去泛素化酶从 H2AK15ub 中去除泛素,导致泛素化染色质的显着积累。由 pUbT12 修饰的染色质无法被 53BP1 访问,但允许同源重组 (HR) 蛋白 RNF169、RAD51 和 BRCA1/BARD1 复合物。染色质环境中 Thr12 处泛素的磷酸化是一种新的组蛋白标记 H2AK15pUbT12,它通过阻碍 53BP1 在受损染色体上的活性来调节 DDR。



























 京公网安备 11010802027423号
京公网安备 11010802027423号