当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Planar-Locked Ru-PNN Catalysts in 1-Phenylethanol Dehydrogenation
Organometallics ( IF 2.5 ) Pub Date : 2020-10-02 , DOI: 10.1021/acs.organomet.0c00327 Paul M. Fanara 1 , Samantha N. MacMillan 2 , David C. Lacy 1
Organometallics ( IF 2.5 ) Pub Date : 2020-10-02 , DOI: 10.1021/acs.organomet.0c00327 Paul M. Fanara 1 , Samantha N. MacMillan 2 , David C. Lacy 1
Affiliation
|
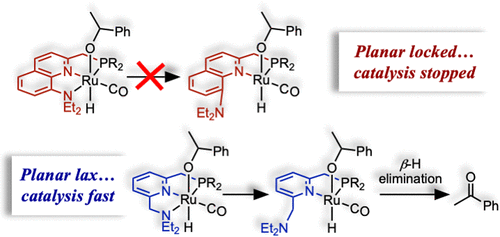
Ru-PNN pincer catalysts of the general form [{PNN}Ru(H)(Cl)(CO)] can dehydrogenate alcohols through inner- and outer-sphere mechanisms, but determining the favored path is challenging. To address this challenge, the following planar-locked quinoline-based PNN ligands, which cannot form key inner-sphere transition states and intermediates, were synthesized: 2-((ditertbutylphosphaneyl)methyl)-N,N-diethylquinolin-8-amine (QNPtBu), 2-((diisopropylphosphaneyl)methyl)-N,N-diethylquinolin-8-amine (QNPiPr), and 2-((diphenylphosphaneyl)methyl)-N,N-diethylquinolin-8-amine (QNPPh). In addition to the quinoline-derived ligands, we also prepared the isoquinoline PNN ligand N-((1-((ditert-butylphosphaneyl)methyl)isoquinolin-3-yl)methyl)-N-ethylethanamine (IsoQNP) and two known picoline- and lutidine-derived ligands 2-((ditert-butylphosphaneyl)methyl)pyridine (PicP) and 2-((ditert-butylphosphaneyl)methyl)-6-methylpyridine (LutP). These six ligands were coordinated to Ru(II) ions to prepare six new complexes of the general formulation [{L}Ru(H)(Cl)(CO)] analogous to Milstein’s PNN catalyst precursor (1PyCl). The X-ray structural, NMR, UV–vis, and FTIR spectroscopic properties of the new complexes are similar to parent complex 1PyCl and were used in catalytic 1-phenylethanol acceptor-less and transfer dehydrogenation. The comparative results demonstrate that 1Py outperforms the other catalysts. DFT reaction profiles were computed for 1Py and the planar-locked catalysts. The results suggest that 1Py has access to a lower-energy inner-sphere path, whereas the planar-locked catalysts can only proceed through a high-energy outer-sphere mechanism and may even get trapped in unreactive alkoxide sinks.
中文翻译:

1-苯基乙醇脱氢中的平面锁定Ru-PNN催化剂
[{PNN} Ru(H)(Cl)(CO)]形式的Ru-PNN钳形催化剂可以通过内球和外球机理将醇脱氢,但确定优选的路径是具有挑战性的。为了应对这一挑战,合成了以下无法形成关键的内球过渡态和中间体的基于平面锁定喹啉的PNN配体:2-((二叔丁基膦酰基)甲基)-N,N-二乙基喹啉-8-胺( QNP TBU),2 - ((diisopropylphosphaneyl)甲基) - ñ,ñ -diethylquinolin -8-胺(QNP的iPr),和2 - ((diphenylphosphaneyl)甲基) - ñ,ñ -diethylquinolin -8-胺(QNP博士)。除了喹啉衍生的配体,我们还制备的异喹啉配体PNN ñ - ((1 - ((二叔-butylphosphaneyl)甲基)异喹啉-3-基)甲基) - ñ -ethylethanamine(IsoQNP)和两个已知的甲基吡啶-和二甲基吡啶的配体2-((二叔丁基膦酰基)甲基)吡啶(PicP)和2-((二叔丁基膦酰基)甲基)-6-甲基吡啶(LutP)。将这六个配体与Ru(II)离子配位,以制备类似于Milstein的PNN催化剂前体(1PyCl)的六种新的通式[{L} Ru(H)(Cl)(CO)]的配合物。新配合物的X射线结构,NMR,UV-vis和FTIR光谱性质类似于母体配合物1PyCl并用于催化1-苯基乙醇无受体和转移脱氢。比较结果表明1Py优于其他催化剂。计算了1Py和平面锁定催化剂的DFT反应曲线。结果表明1Py可以进入较低能量的内球路径,而平面锁定催化剂只能通过高能量的外球机理进行,甚至可能被困在未反应的醇盐池中。
更新日期:2020-10-27
中文翻译:

1-苯基乙醇脱氢中的平面锁定Ru-PNN催化剂
[{PNN} Ru(H)(Cl)(CO)]形式的Ru-PNN钳形催化剂可以通过内球和外球机理将醇脱氢,但确定优选的路径是具有挑战性的。为了应对这一挑战,合成了以下无法形成关键的内球过渡态和中间体的基于平面锁定喹啉的PNN配体:2-((二叔丁基膦酰基)甲基)-N,N-二乙基喹啉-8-胺( QNP TBU),2 - ((diisopropylphosphaneyl)甲基) - ñ,ñ -diethylquinolin -8-胺(QNP的iPr),和2 - ((diphenylphosphaneyl)甲基) - ñ,ñ -diethylquinolin -8-胺(QNP博士)。除了喹啉衍生的配体,我们还制备的异喹啉配体PNN ñ - ((1 - ((二叔-butylphosphaneyl)甲基)异喹啉-3-基)甲基) - ñ -ethylethanamine(IsoQNP)和两个已知的甲基吡啶-和二甲基吡啶的配体2-((二叔丁基膦酰基)甲基)吡啶(PicP)和2-((二叔丁基膦酰基)甲基)-6-甲基吡啶(LutP)。将这六个配体与Ru(II)离子配位,以制备类似于Milstein的PNN催化剂前体(1PyCl)的六种新的通式[{L} Ru(H)(Cl)(CO)]的配合物。新配合物的X射线结构,NMR,UV-vis和FTIR光谱性质类似于母体配合物1PyCl并用于催化1-苯基乙醇无受体和转移脱氢。比较结果表明1Py优于其他催化剂。计算了1Py和平面锁定催化剂的DFT反应曲线。结果表明1Py可以进入较低能量的内球路径,而平面锁定催化剂只能通过高能量的外球机理进行,甚至可能被困在未反应的醇盐池中。











































 京公网安备 11010802027423号
京公网安备 11010802027423号