当前位置:
X-MOL 学术
›
Mol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Arginine-259 of UGT2B7 Confers UDP-Sugar Selectivity
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2020-12-01 , DOI: 10.1124/molpharm.120.000104 Pramod C. Nair , Nuy Chau , Ross A. McKinnon , John O. Miners
Molecular Pharmacology ( IF 3.2 ) Pub Date : 2020-12-01 , DOI: 10.1124/molpharm.120.000104 Pramod C. Nair , Nuy Chau , Ross A. McKinnon , John O. Miners
|
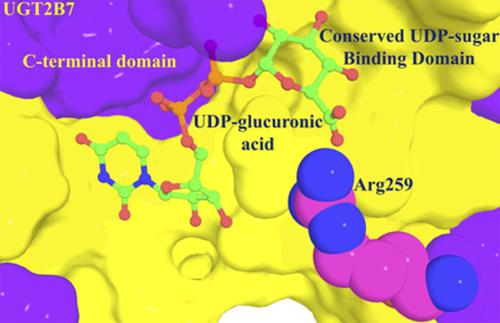
Enzymes of the human UDP-glycosyltransferase (UGT) superfamily typically catalyze the covalent addition of the sugar moiety from a UDP-sugar cofactor to relatively low–molecular weight lipophilic compounds. Although UDP-glucuronic acid (UDP-GlcUA) is most commonly employed as the cofactor by UGT1 and UGT2 family enzymes, UGT2B7 and several other enzymes can use both UDP-GlcUA and UDP-glucose (UDP-Glc), leading to the formation of glucuronide and glucoside conjugates. An investigation of UGT2B7-catalyzed morphine glycosidation indicated that glucuronidation is the principal route of metabolism because the binding affinity of UDP-GlcUA is higher than that of UDP-Glc. Currently, it is unclear which residues in the UGT2B7 cofactor binding domain are responsible for the preferential binding of UDP-GlcUA. Here, molecular dynamics (MD) simulations were performed together with site-directed mutagenesis and enzyme kinetic studies to identify residues within the UGT2B7 binding site responsible for the selective cofactor binding. MD simulations demonstrated that Arg259, which is located within the N-terminal domain, specifically interacts with UDP-GlcUA, whereby the side chain of Arg259 H-bonds and forms a salt bridge with the carboxylate group of glucuronic acid. Consistent with the MD simulations, substitution of Arg259 with Leu resulted in the loss of morphine, 4-methylumbelliferone, and zidovudine glucuronidation activity, but morphine glucosidation was preserved.
中文翻译:

UGT2B7的精氨酸259具有UDP糖选择性
人UDP-糖基转移酶(UGT)超家族的酶通常催化将糖部分从UDP-糖辅因子中共价添加到相对低分子量的亲脂性化合物中。虽然UDP-葡萄糖醛酸(UDP-GlcUA)最常被UGT1和UGT2家族酶用作辅因子,但UGT2B7和其他几种酶可以同时使用UDP-GlcUA和UDP-葡萄糖(UDP-Glc),导致形成葡糖苷酸和葡糖苷结合物。对UGT2B7催化的吗啡糖基化的研究表明,葡糖醛酸化是代谢的主要途径,因为UDP-GlcUA的结合亲和力高于UDP-Glc。目前,尚不清楚UGT2B7辅因子结合结构域中的哪些残基负责UDP-GlcUA的优先结合。这里,分子动力学(MD)模拟与定点诱变和酶动力学研究一起进行,以鉴定UGT2B7结合位点内负责选择性辅因子结合的残基。MD模拟表明,位于N-末端结构域内的Arg259与UDP-GlcUA特异性相互作用,从而Arg259的侧链与葡萄糖醛酸的羧酸酯基团形成氢键并形成盐桥。与MD模拟一致,用Leu取代Arg259导致吗啡,4-甲基伞形酮和齐多夫定葡糖醛酸化活性的丧失,但吗啡糖苷化得以保留。MD模拟表明,位于N-末端结构域内的Arg259与UDP-GlcUA特异性相互作用,从而Arg259的侧链与葡萄糖醛酸的羧酸酯基团形成氢键并形成盐桥。与MD模拟一致,用Leu取代Arg259导致吗啡,4-甲基伞形酮和齐多夫定葡糖醛酸化活性的丧失,但吗啡糖苷化得以保留。MD模拟表明,位于N-末端结构域内的Arg259与UDP-GlcUA特异性相互作用,从而Arg259的侧链与葡萄糖醛酸的羧酸酯基团形成氢键并形成盐桥。与MD模拟一致,用Leu取代Arg259导致吗啡,4-甲基伞形酮和齐多夫定葡糖醛酸化活性的丧失,但吗啡糖苷化得以保留。
更新日期:2020-11-12
中文翻译:

UGT2B7的精氨酸259具有UDP糖选择性
人UDP-糖基转移酶(UGT)超家族的酶通常催化将糖部分从UDP-糖辅因子中共价添加到相对低分子量的亲脂性化合物中。虽然UDP-葡萄糖醛酸(UDP-GlcUA)最常被UGT1和UGT2家族酶用作辅因子,但UGT2B7和其他几种酶可以同时使用UDP-GlcUA和UDP-葡萄糖(UDP-Glc),导致形成葡糖苷酸和葡糖苷结合物。对UGT2B7催化的吗啡糖基化的研究表明,葡糖醛酸化是代谢的主要途径,因为UDP-GlcUA的结合亲和力高于UDP-Glc。目前,尚不清楚UGT2B7辅因子结合结构域中的哪些残基负责UDP-GlcUA的优先结合。这里,分子动力学(MD)模拟与定点诱变和酶动力学研究一起进行,以鉴定UGT2B7结合位点内负责选择性辅因子结合的残基。MD模拟表明,位于N-末端结构域内的Arg259与UDP-GlcUA特异性相互作用,从而Arg259的侧链与葡萄糖醛酸的羧酸酯基团形成氢键并形成盐桥。与MD模拟一致,用Leu取代Arg259导致吗啡,4-甲基伞形酮和齐多夫定葡糖醛酸化活性的丧失,但吗啡糖苷化得以保留。MD模拟表明,位于N-末端结构域内的Arg259与UDP-GlcUA特异性相互作用,从而Arg259的侧链与葡萄糖醛酸的羧酸酯基团形成氢键并形成盐桥。与MD模拟一致,用Leu取代Arg259导致吗啡,4-甲基伞形酮和齐多夫定葡糖醛酸化活性的丧失,但吗啡糖苷化得以保留。MD模拟表明,位于N-末端结构域内的Arg259与UDP-GlcUA特异性相互作用,从而Arg259的侧链与葡萄糖醛酸的羧酸酯基团形成氢键并形成盐桥。与MD模拟一致,用Leu取代Arg259导致吗啡,4-甲基伞形酮和齐多夫定葡糖醛酸化活性的丧失,但吗啡糖苷化得以保留。











































 京公网安备 11010802027423号
京公网安备 11010802027423号