当前位置:
X-MOL 学术
›
Mol. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Domain-Swap Dimerization ofAcanthamoeba castellaniiCYP51 and a Unique Mechanism of Inactivation by Isavuconazole
Molecular Pharmacology ( IF 3.6 ) Pub Date : 2020-12-01 , DOI: 10.1124/molpharm.120.000092 Vandna Sharma 1 , Brian Shing 1 , Lilian Hernandez-Alvarez 1 , Anjan Debnath 1 , Larissa M Podust 2
Molecular Pharmacology ( IF 3.6 ) Pub Date : 2020-12-01 , DOI: 10.1124/molpharm.120.000092 Vandna Sharma 1 , Brian Shing 1 , Lilian Hernandez-Alvarez 1 , Anjan Debnath 1 , Larissa M Podust 2
Affiliation
|
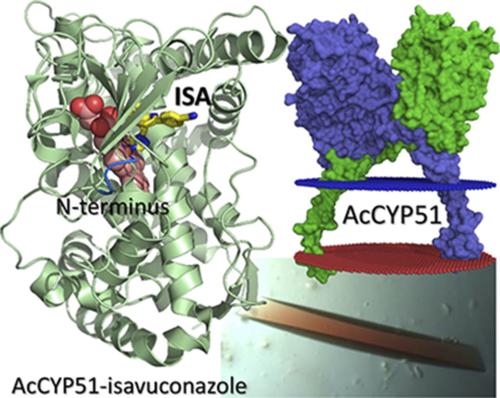
Cytochromes P450 (P450, CYP) metabolize a wide variety of endogenous and exogenous lipophilic molecules, including most drugs. Sterol 14α-demethylase (CYP51) is a target for antifungal drugs known as conazoles. Using X-ray crystallography, we have discovered a domain-swap homodimerization mode in CYP51 from a human pathogen, Acanthamoeba castellanii CYP51 (AcCYP51). Recombinant AcCYP51 with a truncated transmembrane helix was purified as a heterogeneous mixture corresponding to the dimer and monomer units. Spectral analyses of these two populations have shown that the CO-bound ferrous form of the dimeric protein absorbed at 448 nm (catalytically competent form), whereas the monomeric form absorbed at 420 nm (catalytically incompetent form). AcCYP51 dimerized head-to-head via N-termini swapping, resulting in formation of a nonplanar protein-protein interface exceeding 2000 Å2 with a total solvation energy gain of −35.4 kcal/mol. In the dimer, the protomers faced each other through the F and G α-helices, thus blocking the substrate access channel. In the presence of the drugs clotrimazole and isavuconazole, the AcCYP51 drug complexes crystallized as monomers. Although clotrimazole-bound AcCYP51 adopted a typical CYP monomer structure, isavuconazole-bound AcCYP51 failed to refold 74 N-terminal residues. The failure of AcCYP51 to fully refold upon inhibitor binding in vivo would cause an irreversible loss of a structurally aberrant enzyme through proteolytic degradation. This assumption explains the superior potency of isavuconazole against A. castellanii. The dimerization mode observed in this work is compatible with membrane association and may be relevant to other members of the CYP family of biologic, medical, and pharmacological importance.
中文翻译:

卡斯氏棘阿米巴CYP51的结构域交换二聚化和艾沙康唑灭活的独特机制
细胞色素 P450 (P450, CYP) 代谢多种内源性和外源性亲脂性分子,包括大多数药物。甾醇 14 α-脱甲基酶 (CYP51) 是抗真菌药物康唑类药物的靶点。使用 X 射线晶体学,我们发现了来自人类病原体卡斯氏棘阿米巴的CYP51 中的域交换同二聚化模式CYP51 (AcCYP51)。具有截短跨膜螺旋的重组 AcCYP51 被纯化为对应于二聚体和单体单元的异质混合物。这两个群体的光谱分析表明,二聚体蛋白质的 CO 结合亚铁形式在 448 nm 处吸收(催化有效形式),而单体形式在 420 nm 处吸收(催化无效形式)。AcCYP51 通过 N 末端交换头对头二聚化,导致形成超过 2000 Å 2的非平面蛋白质-蛋白质界面,总溶剂化能增益为 -35.4 kcal/mol。在二聚体中,原体通过 F 和 G α-螺旋,从而阻断底物进入通道。在克霉唑和艾沙康唑药物存在下,AcCYP51 药物复合物结晶为单体。尽管克霉唑结合的 AcCYP51 采用了典型的 CYP 单体结构,但艾沙康唑结合的 AcCYP51 未能重新折叠 74 个 N 端残基。AcCYP51 在体内与抑制剂结合后不能完全重折叠,将导致结构异常酶通过蛋白水解降解不可逆地丢失。这一假设解释了艾沙康唑对A. castellanii的优越效力。在这项工作中观察到的二聚化模式与膜结合兼容,并且可能与具有生物学、医学和药理学重要性的 CYP 家族的其他成员有关。
更新日期:2020-11-17
中文翻译:

卡斯氏棘阿米巴CYP51的结构域交换二聚化和艾沙康唑灭活的独特机制
细胞色素 P450 (P450, CYP) 代谢多种内源性和外源性亲脂性分子,包括大多数药物。甾醇 14 α-脱甲基酶 (CYP51) 是抗真菌药物康唑类药物的靶点。使用 X 射线晶体学,我们发现了来自人类病原体卡斯氏棘阿米巴的CYP51 中的域交换同二聚化模式CYP51 (AcCYP51)。具有截短跨膜螺旋的重组 AcCYP51 被纯化为对应于二聚体和单体单元的异质混合物。这两个群体的光谱分析表明,二聚体蛋白质的 CO 结合亚铁形式在 448 nm 处吸收(催化有效形式),而单体形式在 420 nm 处吸收(催化无效形式)。AcCYP51 通过 N 末端交换头对头二聚化,导致形成超过 2000 Å 2的非平面蛋白质-蛋白质界面,总溶剂化能增益为 -35.4 kcal/mol。在二聚体中,原体通过 F 和 G α-螺旋,从而阻断底物进入通道。在克霉唑和艾沙康唑药物存在下,AcCYP51 药物复合物结晶为单体。尽管克霉唑结合的 AcCYP51 采用了典型的 CYP 单体结构,但艾沙康唑结合的 AcCYP51 未能重新折叠 74 个 N 端残基。AcCYP51 在体内与抑制剂结合后不能完全重折叠,将导致结构异常酶通过蛋白水解降解不可逆地丢失。这一假设解释了艾沙康唑对A. castellanii的优越效力。在这项工作中观察到的二聚化模式与膜结合兼容,并且可能与具有生物学、医学和药理学重要性的 CYP 家族的其他成员有关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号