Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Absence of R‐Ras1 and R‐Ras2 causes mitochondrial alterations that trigger axonal degeneration in a hypomyelinating disease model
Glia ( IF 5.4 ) Pub Date : 2020-10-03 , DOI: 10.1002/glia.23917 Berta Alcover-Sanchez 1 , Gonzalo Garcia-Martin 1 , Juan Escudero-Ramirez 1 , Carolina Gonzalez-Riano 2 , Paz Lorenzo 2 , Alfredo Gimenez-Cassina 1 , Laura Formentini 1 , Pedro de la Villa-Polo 3, 4 , Marta P Pereira 1 , Francisco Wandosell 1 , Beatriz Cubelos 1
Glia ( IF 5.4 ) Pub Date : 2020-10-03 , DOI: 10.1002/glia.23917 Berta Alcover-Sanchez 1 , Gonzalo Garcia-Martin 1 , Juan Escudero-Ramirez 1 , Carolina Gonzalez-Riano 2 , Paz Lorenzo 2 , Alfredo Gimenez-Cassina 1 , Laura Formentini 1 , Pedro de la Villa-Polo 3, 4 , Marta P Pereira 1 , Francisco Wandosell 1 , Beatriz Cubelos 1
Affiliation
|
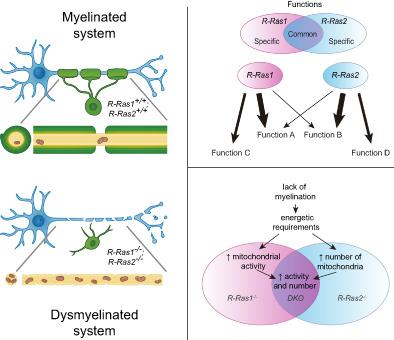
Fast synaptic transmission in vertebrates is critically dependent on myelin for insulation and metabolic support. Myelin is produced by oligodendrocytes (OLs) that maintain multilayered membrane compartments that wrap around axonal fibers. Alterations in myelination can therefore lead to severe pathologies such as multiple sclerosis. Given that hypomyelination disorders have complex etiologies, reproducing clinical symptoms of myelin diseases from a neurological perspective in animal models has been difficult. We recently reported that R‐Ras1−/− and/or R‐Ras2−/− mice, which lack GTPases essential for OL survival and differentiation processes, present different degrees of hypomyelination in the central nervous system with a compounded hypomyelination in double knockout (DKO) mice. Here, we discovered that the loss of R‐Ras1 and/or R‐Ras2 function is associated with aberrant myelinated axons with increased numbers of mitochondria, and a disrupted mitochondrial respiration that leads to increased reactive oxygen species levels. Consequently, aberrant myelinated axons are thinner with cytoskeletal phosphorylation patterns typical of axonal degeneration processes, characteristic of myelin diseases. Although we observed different levels of hypomyelination in a single mutant mouse, the combined loss of function in DKO mice lead to a compromised axonal integrity, triggering the loss of visual function. Our findings demonstrate that the loss of R‐Ras function reproduces several characteristics of hypomyelinating diseases, and we therefore propose that R‐Ras1−/− and R‐Ras2−/− neurological models are valuable approaches for the study of these myelin pathologies.
中文翻译:

R-Ras1 和 R-Ras2 的缺失导致线粒体改变,在髓鞘形成疾病模型中引发轴突变性
脊椎动物的快速突触传递严重依赖于髓鞘的绝缘和代谢支持。髓磷脂由少突胶质细胞 (OL) 产生,这些细胞维持包裹在轴突纤维周围的多层膜隔室。因此,髓鞘形成的改变会导致严重的疾病,例如多发性硬化症。鉴于髓鞘形成障碍具有复杂的病因,在动物模型中从神经学角度再现髓鞘疾病的临床症状一直很困难。我们最近报道了R-Ras1 -/-和/或R-Ras2 -/-缺乏对 OL 存活和分化过程至关重要的 GTP 酶的小鼠,在中枢神经系统中呈现不同程度的髓鞘形成,在双敲除 ( DKO ) 小鼠中存在复合髓鞘形成。在这里,我们发现 R-Ras1 和/或 R-Ras2 功能的丧失与线粒体数量增加的异常有髓轴突和导致活性氧水平增加的线粒体呼吸中断有关。因此,异常的有髓轴突更薄,具有典型的轴突变性过程的细胞骨架磷酸化模式,这是髓鞘疾病的特征。尽管我们在单个突变小鼠中观察到不同程度的髓鞘形成,但DKO中的综合功能丧失小鼠会导致轴突完整性受损,从而引发视觉功能的丧失。我们的研究结果表明,R-Ras 功能的丧失再现了髓鞘形成疾病的几个特征,因此我们建议R-Ras1 -/-和R-Ras2 -/-神经学模型是研究这些髓鞘病变的有价值的方法。
更新日期:2020-10-03
中文翻译:

R-Ras1 和 R-Ras2 的缺失导致线粒体改变,在髓鞘形成疾病模型中引发轴突变性
脊椎动物的快速突触传递严重依赖于髓鞘的绝缘和代谢支持。髓磷脂由少突胶质细胞 (OL) 产生,这些细胞维持包裹在轴突纤维周围的多层膜隔室。因此,髓鞘形成的改变会导致严重的疾病,例如多发性硬化症。鉴于髓鞘形成障碍具有复杂的病因,在动物模型中从神经学角度再现髓鞘疾病的临床症状一直很困难。我们最近报道了R-Ras1 -/-和/或R-Ras2 -/-缺乏对 OL 存活和分化过程至关重要的 GTP 酶的小鼠,在中枢神经系统中呈现不同程度的髓鞘形成,在双敲除 ( DKO ) 小鼠中存在复合髓鞘形成。在这里,我们发现 R-Ras1 和/或 R-Ras2 功能的丧失与线粒体数量增加的异常有髓轴突和导致活性氧水平增加的线粒体呼吸中断有关。因此,异常的有髓轴突更薄,具有典型的轴突变性过程的细胞骨架磷酸化模式,这是髓鞘疾病的特征。尽管我们在单个突变小鼠中观察到不同程度的髓鞘形成,但DKO中的综合功能丧失小鼠会导致轴突完整性受损,从而引发视觉功能的丧失。我们的研究结果表明,R-Ras 功能的丧失再现了髓鞘形成疾病的几个特征,因此我们建议R-Ras1 -/-和R-Ras2 -/-神经学模型是研究这些髓鞘病变的有价值的方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号