当前位置:
X-MOL 学术
›
Immunology
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Improved prediction of HLA antigen presentation hotspots: Applications for immunogenicity risk assessment of therapeutic proteins
Immunology ( IF 4.9 ) Pub Date : 2020-10-03 , DOI: 10.1111/imm.13274 Anders Steenholdt Attermann 1 , Carolina Barra 1 , Birkir Reynisson 1 , Heidi Schiøler Schultz 2 , Ulrike Leurs 2 , Kasper Lamberth 2 , Morten Nielsen 1, 3
Immunology ( IF 4.9 ) Pub Date : 2020-10-03 , DOI: 10.1111/imm.13274 Anders Steenholdt Attermann 1 , Carolina Barra 1 , Birkir Reynisson 1 , Heidi Schiøler Schultz 2 , Ulrike Leurs 2 , Kasper Lamberth 2 , Morten Nielsen 1, 3
Affiliation
|
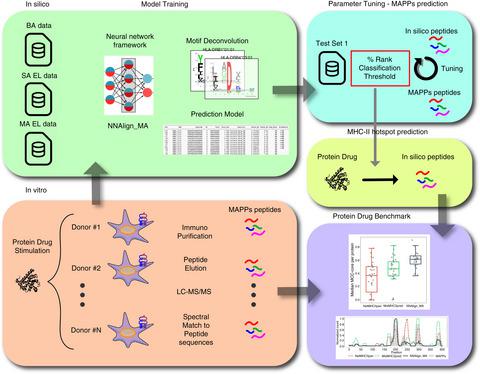
Immunogenicity risk assessment is a critical element in protein drug development. Currently, the risk assessment is most often performed using MHC‐associated peptide proteomics (MAPPs) and/or T‐cell activation assays. However, this is a highly costly procedure that encompasses limited sensitivity imposed by sample sizes, the MHC repertoire of the tested donor cohort and the experimental procedures applied. Recent work has suggested that these techniques could be complemented by accurate, high‐throughput and cost‐effective prediction of in silico models. However, this work covered a very limited set of therapeutic proteins and eluted ligand (EL) data. Here, we resolved these limitations by showcasing, in a broader setting, the versatility of in silico models for assessment of protein drug immunogenicity. A method for prediction of MHC class II antigen presentation was developed on the hereto largest available mass spectrometry (MS) HLA‐DR EL data set. Using independent test sets, the performance of the method for prediction of HLA‐DR antigen presentation hotspots was benchmarked. In particular, the method was showcased on a set of protein sequences including four therapeutic proteins and demonstrated to accurately predict the experimental MS hotspot regions at a significantly lower false‐positive rate compared with other methods. This gain in performance was particularly pronounced when compared to the NetMHCIIpan‐3.2 method trained on binding affinity data. These results suggest that in silico methods trained on MS HLA EL data can effectively and accurately be used to complement MAPPs assays for the risk assessment of protein drugs.
中文翻译:

改进 HLA 抗原呈递热点的预测:治疗性蛋白免疫原性风险评估的应用
免疫原性风险评估是蛋白质药物开发的关键要素。目前,风险评估最常使用 MHC 相关肽蛋白质组学 (MAPP) 和/或 T 细胞活化测定法进行。然而,这是一个成本高昂的过程,包括样本大小、测试供体队列的 MHC 曲目和应用的实验程序所施加的有限敏感性。最近的工作表明,这些技术可以通过对计算机模型的准确、高通量和经济高效的预测进行补充。然而,这项工作涵盖了一组非常有限的治疗性蛋白质和洗脱配体 (EL) 数据。在这里,我们通过在更广泛的环境中展示用于评估蛋白质药物免疫原性的计算机模型的多功能性来解决这些限制。在迄今为止最大的可用质谱 (MS) HLA-DR EL 数据集上开发了一种预测 MHC II 类抗原呈递的方法。使用独立的测试集,对预测 HLA-DR 抗原呈递热点的方法的性能进行了基准测试。特别是,该方法在一组蛋白质序列上进行了展示,其中包括四种治疗性蛋白质,并证明与其他方法相比,该方法可以以显着降低的假阳性率准确预测实验性 MS 热点区域。与在结合亲和力数据上训练的 NetMHCIIpan-3.2 方法相比,这种性能提升尤其明显。这些结果表明,在 MS HLA EL 数据上训练的计算机方法可以有效和准确地用于补充 MAPP 分析,以进行蛋白质药物的风险评估。
更新日期:2020-10-03
中文翻译:

改进 HLA 抗原呈递热点的预测:治疗性蛋白免疫原性风险评估的应用
免疫原性风险评估是蛋白质药物开发的关键要素。目前,风险评估最常使用 MHC 相关肽蛋白质组学 (MAPP) 和/或 T 细胞活化测定法进行。然而,这是一个成本高昂的过程,包括样本大小、测试供体队列的 MHC 曲目和应用的实验程序所施加的有限敏感性。最近的工作表明,这些技术可以通过对计算机模型的准确、高通量和经济高效的预测进行补充。然而,这项工作涵盖了一组非常有限的治疗性蛋白质和洗脱配体 (EL) 数据。在这里,我们通过在更广泛的环境中展示用于评估蛋白质药物免疫原性的计算机模型的多功能性来解决这些限制。在迄今为止最大的可用质谱 (MS) HLA-DR EL 数据集上开发了一种预测 MHC II 类抗原呈递的方法。使用独立的测试集,对预测 HLA-DR 抗原呈递热点的方法的性能进行了基准测试。特别是,该方法在一组蛋白质序列上进行了展示,其中包括四种治疗性蛋白质,并证明与其他方法相比,该方法可以以显着降低的假阳性率准确预测实验性 MS 热点区域。与在结合亲和力数据上训练的 NetMHCIIpan-3.2 方法相比,这种性能提升尤其明显。这些结果表明,在 MS HLA EL 数据上训练的计算机方法可以有效和准确地用于补充 MAPP 分析,以进行蛋白质药物的风险评估。











































 京公网安备 11010802027423号
京公网安备 11010802027423号