当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Silica based ionogels: interface effects with aprotic and protic ionic liquids with lithium
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-10-02 , DOI: 10.1039/d0cp03599h Angélique Marie 1, 2, 3, 4, 5 , Bilel Said 2, 6, 7, 8, 9 , Anne Galarneau 2, 6, 7, 8, 9 , Timo Stettner 10, 11, 12, 13, 14 , Andrea Balducci 10, 11, 12, 13, 14 , Maxime Bayle 1, 2, 3, 4, 5 , Bernard Humbert 1, 2, 3, 4, 5 , Jean Le Bideau 1, 2, 3, 4, 5
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2020-10-02 , DOI: 10.1039/d0cp03599h Angélique Marie 1, 2, 3, 4, 5 , Bilel Said 2, 6, 7, 8, 9 , Anne Galarneau 2, 6, 7, 8, 9 , Timo Stettner 10, 11, 12, 13, 14 , Andrea Balducci 10, 11, 12, 13, 14 , Maxime Bayle 1, 2, 3, 4, 5 , Bernard Humbert 1, 2, 3, 4, 5 , Jean Le Bideau 1, 2, 3, 4, 5
Affiliation
|
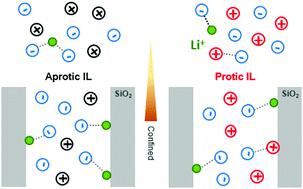
In the frame of the development of solid ionogel electrolytes with enhanced ion transport properties, this paper investigates ionogel systems constituted by ∼80 wt% of ionic liquids (ILs) confined in meso-/macroporous silica monolith materials. The anion–cation coordination for two closely related ILs, either aprotic (AIL) butylmethylpyrrolidinium or protic (PIL) butylpyrrolidinium, both with bis(trifluoromethylsulfonyl)imide (TFSI) anions, with and without lithium cations, is studied in depth. The ILs are confined within silica with well-defined mesoporosities (8 to 16 nm). The effects of this confinement, onto melting points, onto conductivity followed by impedance spectroscopy, and onto lithium–TFSI coordination followed by Raman spectroscopy, are presented. Opposite effects have been observed on the melting temperature: it increased for the AIL (+2 °C) upon confinement, while it decreased for the PIL (−2 °C). With lithium, the confinement led to an increase of the melting temperature (+1 °C) for the PIL and AIL. Regarding ionic conductivities, a relative maximum was observed at 40 °C for a mesopore diameter of 10 nm for the AIL with 0.5 M lithium, while it was not clearly visible for the PIL. These differences are discussed in view of the charge balance at the interface between silanols and ILs: the presence of a PIL, contrary to an AIL, is expected to modify the acidity of the silica. Raman data showed that the coordination number of lithium by TFSI is reduced upon AIL confinement, although this was not observed for PILs. At last, this work highlights the impact of the acidity of a PIL on the chemistry occurring at the interface of the host network within ionogels.
中文翻译:

二氧化硅基离子凝胶:质子惰性和质子离子液体与锂的界面效应
在开发具有增强的离子传输性能的固体离子凝胶电解质的框架中,本文研究了由约80 wt%的离子液体(ILs)限制在中/大型二氧化硅整体材料中构成的离子凝胶体系。深入研究了两个密切相关的ILs的阴离子-阳离子配位,即质子传递(AIL)丁基甲基吡咯烷鎓或质子传递(PIL)丁基吡咯烷鎓,同时带有和不带有锂阳离子的双(三氟甲基磺酰基)酰亚胺(TFSI)阴离子。IL被限制在具有明确介孔(8至16 nm)的二氧化硅内。提出了这种限制对熔点,电导率和阻抗谱的影响以及对锂-TFSI配位作用和拉曼光谱的影响。在熔化温度上观察到相反的影响:封闭时,AIL(+2°C)增加,而PIL(-2°C)减少。对于锂,这种限制导致PIL和AIL的熔化温度(+1°C)升高。关于离子电导率,对于带有0.5 M锂的AIL,在40°C的中孔直径为10 nm时观察到相对最大值,而对于PIL则不明显。鉴于硅烷醇和IL之间的界面处的电荷平衡,讨论了这些差异:与AIL相反,PIL的存在有望改变二氧化硅的酸度。拉曼数据显示,在AIL限制下,TFSI可使锂的配位数降低,尽管PIL并未观察到。最后,
更新日期:2020-10-20
中文翻译:

二氧化硅基离子凝胶:质子惰性和质子离子液体与锂的界面效应
在开发具有增强的离子传输性能的固体离子凝胶电解质的框架中,本文研究了由约80 wt%的离子液体(ILs)限制在中/大型二氧化硅整体材料中构成的离子凝胶体系。深入研究了两个密切相关的ILs的阴离子-阳离子配位,即质子传递(AIL)丁基甲基吡咯烷鎓或质子传递(PIL)丁基吡咯烷鎓,同时带有和不带有锂阳离子的双(三氟甲基磺酰基)酰亚胺(TFSI)阴离子。IL被限制在具有明确介孔(8至16 nm)的二氧化硅内。提出了这种限制对熔点,电导率和阻抗谱的影响以及对锂-TFSI配位作用和拉曼光谱的影响。在熔化温度上观察到相反的影响:封闭时,AIL(+2°C)增加,而PIL(-2°C)减少。对于锂,这种限制导致PIL和AIL的熔化温度(+1°C)升高。关于离子电导率,对于带有0.5 M锂的AIL,在40°C的中孔直径为10 nm时观察到相对最大值,而对于PIL则不明显。鉴于硅烷醇和IL之间的界面处的电荷平衡,讨论了这些差异:与AIL相反,PIL的存在有望改变二氧化硅的酸度。拉曼数据显示,在AIL限制下,TFSI可使锂的配位数降低,尽管PIL并未观察到。最后,











































 京公网安备 11010802027423号
京公网安备 11010802027423号