当前位置:
X-MOL 学术
›
J. Neurochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
PKC- and PKA-dependent phosphorylation modulates TREK-1 function in naïve and neuropathic rats
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-10-02 , DOI: 10.1111/jnc.15204 Guadalupe García 1 , Karina A Méndez-Reséndiz 1 , Norma Oviedo 2 , Janet Murbartián 1
Journal of Neurochemistry ( IF 4.2 ) Pub Date : 2020-10-02 , DOI: 10.1111/jnc.15204 Guadalupe García 1 , Karina A Méndez-Reséndiz 1 , Norma Oviedo 2 , Janet Murbartián 1
Affiliation
|
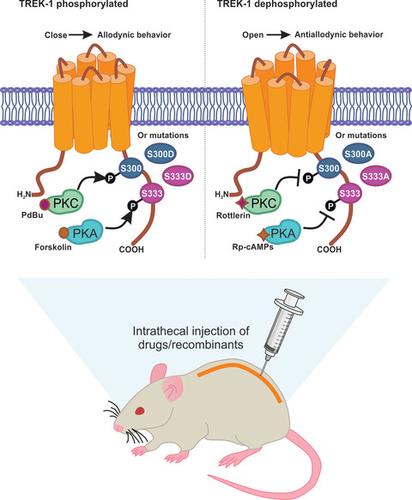
PKC and PKA phosphorylation inhibit TREK-1 channels downstream of Gs protein-coupled receptor activation in vitro. However, the role of phosphorylation of TREK-1 in neuropathic pain is unknown. The purpose of this study was to investigate whether altered TREK-1 channel function by PKA and PKC modulators contributes to antiallodynia in neuropathic rats. Furthermore, we investigated if the in vitro described sites for PKC and PKA phosphorylation (S300 and S333, respectively) participate in the modulation of TREK-1 in naïve and neuropathic rats. L5/L6 spinal nerve ligation (SNL) induced tactile allodynia. Intrathecal injection of BL-1249 (TREK-1 activator) reversed nerve injury-induced tactile allodynia, whereas spadin (TREK-1 blocker) produced tactile allodynia in naïve rats and reversed the antiallodynic effect induced by BL-1249 in neuropathic rats. Intrathecal administration of rottlerin or Rp-cAMPs (PKC and PKA inhibitors, respectively) enhanced the antiallodynia observed with BL-1249 in neuropathic rats. In contrast, pretreatment with PdBu or forskolin (PKC and PKA activators, respectively) reduced the BL-1249-induced antiallodynia. Intrathecal injection of two high-activity TREK-1 recombinant channels, using a in vivo transfection method with lipofectamine, with mutations at PKC/PKA phosphosites (S300A and S333A) reversed tactile allodynia in neuropathic rats, with no effect in naïve rats. In contrast, transfection of two low-activity TREK-1 recombinant channels with phosphomimetic mutations at those sites (S300D and S333D) produced tactile allodynia in naïve rats and interfered with antiallodynic effects of rottlerin/BL-1249 or Rp-cAMPs/BL-1249. Data suggest that TREK-1 channel activity can be dynamically tuned in vivo by PKC/PKA to provoke allodynia and modulate its antiallodynic role in neuropathic pain.
中文翻译:

PKC 和 PKA 依赖性磷酸化调节幼稚和神经病大鼠的 TREK-1 功能
PKC和PKA磷酸化抑制的G下游TREK-1通道小号蛋白偶联受体激活在体外. 然而,TREK-1 磷酸化在神经性疼痛中的作用尚不清楚。本研究的目的是研究 PKA 和 PKC 调节剂改变的 TREK-1 通道功能是否有助于神经性大鼠的抗异常性疼痛。此外,我们研究了体外描述的 PKC 和 PKA 磷酸化位点(分别为 S300 和 S333)是否参与了幼稚和神经病大鼠中 TREK-1 的调节。L5/L6 脊神经结扎术 (SNL) 诱发的触觉异常性疼痛。鞘内注射 BL-1249(TREK-1 激活剂)可逆转神经损伤引起的触觉异常性疼痛,而斯巴丁(TREK-1 阻滞剂)可在幼稚大鼠中产生触觉异常性疼痛,并逆转 BL-1249 在神经性大鼠中引起的抗异常性疼痛作用。Rottlerin 或 Rp-cAMP(PKC 和 PKA 抑制剂,分别)增强了用 BL-1249 在神经性大鼠中观察到的抗异常性疼痛。相比之下,用 PdBu 或毛喉素(分别为 PKC 和 PKA 激活剂)预处理可减少 BL-1249 诱导的抗异常性疼痛。鞘内注射两种高活性 TREK-1 重组通道,使用 lipofectamine 的体内转染方法,在 PKC/PKA 磷酸位点(S300A 和 S333A)发生突变,逆转了神经性大鼠的触觉异常性疼痛,对幼稚大鼠没有影响。相比之下,在这些位点(S300D 和 S333D)转染两个低活性 TREK-1 重组通道,在这些位点(S300D 和 S333D)产生了触觉异常性疼痛,并干扰了 Rottlerin/BL-1249 或 Rp-cAMPs/BL-1249 的抗异常性疼痛作用.
更新日期:2020-10-02
中文翻译:

PKC 和 PKA 依赖性磷酸化调节幼稚和神经病大鼠的 TREK-1 功能
PKC和PKA磷酸化抑制的G下游TREK-1通道小号蛋白偶联受体激活在体外. 然而,TREK-1 磷酸化在神经性疼痛中的作用尚不清楚。本研究的目的是研究 PKA 和 PKC 调节剂改变的 TREK-1 通道功能是否有助于神经性大鼠的抗异常性疼痛。此外,我们研究了体外描述的 PKC 和 PKA 磷酸化位点(分别为 S300 和 S333)是否参与了幼稚和神经病大鼠中 TREK-1 的调节。L5/L6 脊神经结扎术 (SNL) 诱发的触觉异常性疼痛。鞘内注射 BL-1249(TREK-1 激活剂)可逆转神经损伤引起的触觉异常性疼痛,而斯巴丁(TREK-1 阻滞剂)可在幼稚大鼠中产生触觉异常性疼痛,并逆转 BL-1249 在神经性大鼠中引起的抗异常性疼痛作用。Rottlerin 或 Rp-cAMP(PKC 和 PKA 抑制剂,分别)增强了用 BL-1249 在神经性大鼠中观察到的抗异常性疼痛。相比之下,用 PdBu 或毛喉素(分别为 PKC 和 PKA 激活剂)预处理可减少 BL-1249 诱导的抗异常性疼痛。鞘内注射两种高活性 TREK-1 重组通道,使用 lipofectamine 的体内转染方法,在 PKC/PKA 磷酸位点(S300A 和 S333A)发生突变,逆转了神经性大鼠的触觉异常性疼痛,对幼稚大鼠没有影响。相比之下,在这些位点(S300D 和 S333D)转染两个低活性 TREK-1 重组通道,在这些位点(S300D 和 S333D)产生了触觉异常性疼痛,并干扰了 Rottlerin/BL-1249 或 Rp-cAMPs/BL-1249 的抗异常性疼痛作用.











































 京公网安备 11010802027423号
京公网安备 11010802027423号