当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One‐Shot Radical Cross Coupling Between Benzyl Alcohols and Alkenyl Halides Using Ni/Ti/Mn System
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-01 , DOI: 10.1002/adsc.202000945 Takuya Suga 1 , Yuuki Takahashi 1 , Yutaka Ukaji 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-01 , DOI: 10.1002/adsc.202000945 Takuya Suga 1 , Yuuki Takahashi 1 , Yutaka Ukaji 1
Affiliation
|
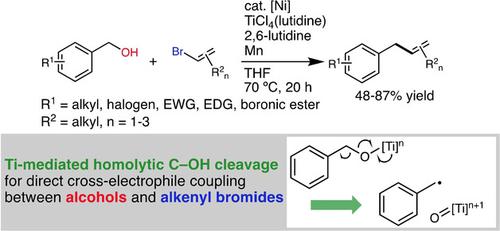
A “one‐shot” cross coupling between benzyl alcohols and alkenyl halides has been established. A combination of low‐valent Ti‐mediated C−OH homolysis and the prominent chemistry of Ni‐based radical catalysis afforded the desired cross‐coupled product with good efficiency. The reaction proceeded regardless of the electronic property of benzyl alcohols, and Ar−B bond remained intact throughout the reaction. Alkenyl bromides with various substitution patterns were applicable to this reaction. Attempts for utilizing sterically demanding tri‐substituted alkenes indicated that the steric hinderance mainly inhibited the radical‐trapping by Ni species. This reaction can be a simple and efficient strategy for synthesizing densely substituted allylbenzene derivatives.
中文翻译:

Ni / Ti / Mn体系在苄醇与烯基卤化物之间的单发自由基交叉偶联
在苄醇和烯基卤化物之间建立了“一次性”交叉偶联。低价Ti介导的C-OH均解反应和突出的Ni基自由基催化化学作用相结合,提供了所需的交叉偶联产物,效率很高。无论苄醇的电子性质如何,反应都会进行,并且Ar-B键在整个反应过程中保持完整。具有各种取代方式的烯基溴化物可用于该反应。尝试利用具有空间需求的三取代烯烃,表明空间位阻主要抑制了Ni物种的自由基捕获。该反应可以是合成密集取代的烯丙基苯衍生物的简单有效的策略。
更新日期:2020-10-01
中文翻译:

Ni / Ti / Mn体系在苄醇与烯基卤化物之间的单发自由基交叉偶联
在苄醇和烯基卤化物之间建立了“一次性”交叉偶联。低价Ti介导的C-OH均解反应和突出的Ni基自由基催化化学作用相结合,提供了所需的交叉偶联产物,效率很高。无论苄醇的电子性质如何,反应都会进行,并且Ar-B键在整个反应过程中保持完整。具有各种取代方式的烯基溴化物可用于该反应。尝试利用具有空间需求的三取代烯烃,表明空间位阻主要抑制了Ni物种的自由基捕获。该反应可以是合成密集取代的烯丙基苯衍生物的简单有效的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号