当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Formal Ring Contraction of Cyclic N‐Sulfonamides via C−N Bond Cleavage and α‐Amination by Oxidation of Halides
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-01 , DOI: 10.1002/adsc.202001034 Yuna Nishiguchi 1 , Akihiko Tomizuka 1 , Katsuhiko Moriyama 2
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-01 , DOI: 10.1002/adsc.202001034 Yuna Nishiguchi 1 , Akihiko Tomizuka 1 , Katsuhiko Moriyama 2
Affiliation
|
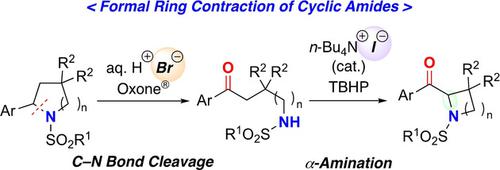
An oxidative C−N bond cleavage reaction of substituted cyclic sulfonamides that proceeds via oxidation of bromide ion in hydrogen bromide solution was developed to furnish N‐sulfonamide‐protected acyclic amino ketones in high yields. The subsequent α‐amination of the amino ketones via the oxidation of iodide in tetrabutylammonium iodide provided 2‐acyl cyclic sulfonamides in good yields. The combination of these transformations involving the oxidation of halides resulted in a formal ring contraction of substituted cyclic amides.
中文翻译:

环状N-磺酰胺通过C-N键断裂和卤化物氧化引起的α-胺化的形式环收缩
通过在溴化氢溶液中溴离子的氧化进行的取代的环磺酰胺的C-N键氧化裂解反应被开发出来,以高产率提供N-磺酰胺保护的无环氨基酮。随后,碘化物在四丁基碘化铵中通过碘化物的氧化作用而进行氨基酮的α-胺化反应,可得到高产率的2-酰基环磺酰胺。这些涉及卤化物氧化的转化的组合导致取代的环状酰胺的正式环收缩。
更新日期:2020-12-08
中文翻译:

环状N-磺酰胺通过C-N键断裂和卤化物氧化引起的α-胺化的形式环收缩
通过在溴化氢溶液中溴离子的氧化进行的取代的环磺酰胺的C-N键氧化裂解反应被开发出来,以高产率提供N-磺酰胺保护的无环氨基酮。随后,碘化物在四丁基碘化铵中通过碘化物的氧化作用而进行氨基酮的α-胺化反应,可得到高产率的2-酰基环磺酰胺。这些涉及卤化物氧化的转化的组合导致取代的环状酰胺的正式环收缩。











































 京公网安备 11010802027423号
京公网安备 11010802027423号