当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Divergent Halogenation Pathways of 2,2‐Dichlorobut‐3‐yn‐1‐ols to 3‐Chloro‐4‐Iodofurans and α‐Chloro‐γ‐Iodoallenes: Electrophilic versus Pd(II)‐Catalyzed Halogenation Strategies
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-01 , DOI: 10.1002/adsc.202001033 Kyungsoo Oh 1 , Hun Young Kim 1 , Mohamed Ahmed Abozeid 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-10-01 , DOI: 10.1002/adsc.202001033 Kyungsoo Oh 1 , Hun Young Kim 1 , Mohamed Ahmed Abozeid 1
Affiliation
|
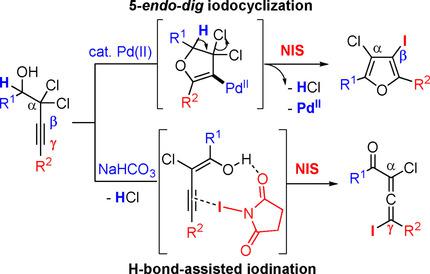
Divergent halogenation pathways of 2,2‐dichlorobut‐3‐yn‐1‐ols have been developed to give 3,4‐dihalofurans and 1,3‐dihaloallenyl ketones in good to excellent yields. Thus, the readily accessible 2,2‐dichlorobut‐3‐yn‐1‐ols were treated with the electrophilic halogen source to provide the 1,3‐dihalogen‐substituted allenyl ketones, whereas the use of Pd(II) catalyst promoted the oxypalladation followed by the electrophilic halogenation to give 3,4‐dihalogen‐substituted furan derivatives. The synthetic utility of 3,4‐dihalofurans and 1,3‐dihaloallenyl ketones is demonstrated in the sequential Suzuki cross coupling strategies.
中文翻译:

2,2-二氯丁-3-yn-1-醇到3-氯-4-碘呋喃和α-氯γ-碘丙二烯的不同卤化途径:亲电与Pd(II)催化的卤化策略
已开发出2,2-二氯丁3 -yn-1-醇的不同卤化途径,从而以良好或极好的收率得到3,4-二卤呋喃和1,3-二卤代烯基酮。因此,使用亲电子卤素源处理易于获得的2,2-二氯丁-3-yn-1-醇,以提供1,3-二卤素取代的烯基酮,而使用Pd(II)催化剂则促进了氧pal然后进行亲电卤化,得到3,4-二卤代呋喃衍生物。在顺序的Suzuki交叉偶联策略中证明了3,4-二卤呋喃和1,3-二卤代烯基酮的合成效用。
更新日期:2020-12-08
中文翻译:

2,2-二氯丁-3-yn-1-醇到3-氯-4-碘呋喃和α-氯γ-碘丙二烯的不同卤化途径:亲电与Pd(II)催化的卤化策略
已开发出2,2-二氯丁3 -yn-1-醇的不同卤化途径,从而以良好或极好的收率得到3,4-二卤呋喃和1,3-二卤代烯基酮。因此,使用亲电子卤素源处理易于获得的2,2-二氯丁-3-yn-1-醇,以提供1,3-二卤素取代的烯基酮,而使用Pd(II)催化剂则促进了氧pal然后进行亲电卤化,得到3,4-二卤代呋喃衍生物。在顺序的Suzuki交叉偶联策略中证明了3,4-二卤呋喃和1,3-二卤代烯基酮的合成效用。










































 京公网安备 11010802027423号
京公网安备 11010802027423号