当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Multiple crystal forms of human MacroD2
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-10-02 , DOI: 10.1107/s2053230x20011309 Sarah Wazir 1 , Mirko M Maksimainen 1 , Lari Lehtiö 1
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-10-02 , DOI: 10.1107/s2053230x20011309 Sarah Wazir 1 , Mirko M Maksimainen 1 , Lari Lehtiö 1
Affiliation
|
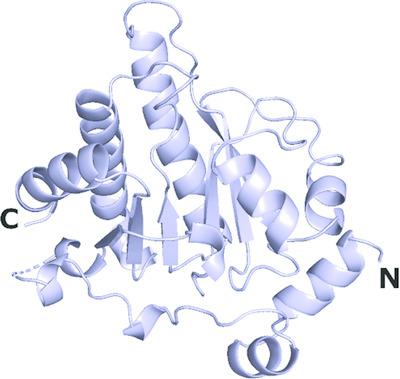
MacroD2 is one of the three human macrodomain proteins characterized by their protein‐linked mono‐ADP‐ribosyl‐hydrolyzing activity. MacroD2 is a single‐domain protein that contains a deep ADP‐ribose‐binding groove. In this study, new crystallization conditions for MacroD2 were found and three crystal structures of human MacroD2 in the apo state were solved in space groups P41212, P43212 and P43, and refined at 1.75, 1.90 and 1.70 Å resolution, respectively. Structural comparison of the apo crystal structures with the previously reported crystal structure of MacroD2 in complex with ADP‐ribose revealed conformational changes in the side chains of Val101, Ile189 and Phe224 induced by the binding of ADP‐ribose in the active site. These conformational variations may potentially facilitate design efforts of a MacroD2 inhibitor.
中文翻译:

人 MacroD2 的多种晶型
MacroD2 是三种人类宏结构域蛋白之一,其特征在于其蛋白连接的单 ADP 核糖基水解活性。 MacroD2 是一种单结构域蛋白,包含深 ADP 核糖结合沟。本研究发现了MacroD2新的结晶条件,并在空间群P 4 1 2 1 2、 P 4 3 2 1 2和P 4 3中解析了人MacroD2在apo状态下的三种晶体结构,并细化至1.75,分辨率分别为 1.90 和 1.70 Å。将 apo 晶体结构与先前报道的 MacroD2 与 ADP-核糖复合物的晶体结构进行结构比较,揭示了由 ADP-核糖在活性位点的结合引起的 Val101、Ile189 和 Phe224 侧链的构象变化。这些构象变化可能有利于 MacroD2 抑制剂的设计工作。
更新日期:2020-10-02
中文翻译:

人 MacroD2 的多种晶型
MacroD2 是三种人类宏结构域蛋白之一,其特征在于其蛋白连接的单 ADP 核糖基水解活性。 MacroD2 是一种单结构域蛋白,包含深 ADP 核糖结合沟。本研究发现了MacroD2新的结晶条件,并在空间群P 4 1 2 1 2、 P 4 3 2 1 2和P 4 3中解析了人MacroD2在apo状态下的三种晶体结构,并细化至1.75,分辨率分别为 1.90 和 1.70 Å。将 apo 晶体结构与先前报道的 MacroD2 与 ADP-核糖复合物的晶体结构进行结构比较,揭示了由 ADP-核糖在活性位点的结合引起的 Val101、Ile189 和 Phe224 侧链的构象变化。这些构象变化可能有利于 MacroD2 抑制剂的设计工作。











































 京公网安备 11010802027423号
京公网安备 11010802027423号