当前位置:
X-MOL 学术
›
Acta Cryst. F
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structure of XCC3289 from Xanthomonas campestris: homology with the N‐terminal substrate‐binding domain of Lon peptidase
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-10-02 , DOI: 10.1107/s2053230x20011875 Rahul Singh 1 , Sonali Deshmukh 1 , Ashwani Kumar 1 , Venuka Durani Goyal 1 , Ravindra D Makde 1
Acta Crystallographica Section F ( IF 1.1 ) Pub Date : 2020-10-02 , DOI: 10.1107/s2053230x20011875 Rahul Singh 1 , Sonali Deshmukh 1 , Ashwani Kumar 1 , Venuka Durani Goyal 1 , Ravindra D Makde 1
Affiliation
|
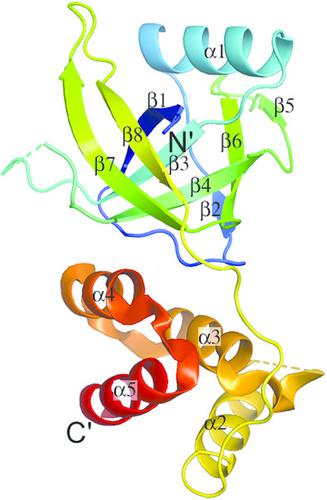
LonA peptidase is a major component of the protein quality‐control mechanism in both prokaryotes and the organelles of eukaryotes. Proteins homologous to the N‐terminal domain of LonA peptidase, but lacking its other domains, are conserved in several phyla of prokaryotes, including the Xanthomonadales order. However, the function of these homologous proteins (LonNTD‐like proteins) is not known. Here, the crystal structure of the LonNTD‐like protein from Xanthomonas campestris (XCC3289; UniProt Q8P5P7) is reported at 2.8 Å resolution. The structure was solved by molecular replacement and contains one polypeptide in the asymmetric unit. The structure was refined to an Rfree of 29%. The structure of XCC3289 consists of two domains joined by a long loop. The N‐terminal domain (residues 1–112) consists of an α‐helix surrounded by β‐sheets, whereas the C‐terminal domain (residues 123–193) is an α‐helical bundle. The fold and spatial orientation of the two domains closely resembles those of the N‐terminal domains of the LonA peptidases from Escherichia coli and Mycobacterium avium. The structure is also similar to that of cereblon, a substrate‐recognizing component of the E3 ubiquitin ligase complex. The N‐terminal domains of both LonA and cereblon are known to be involved in specific protein–protein interactions. This structural analysis suggests that XCC3289 and other LonNTD‐like proteins might also be capable of such protein–protein interactions.
中文翻译:

野油菜黄单胞菌 XCC3289 的晶体结构:与 Lon 肽酶 N 端底物结合域的同源性
LonA 肽酶是原核生物和真核生物细胞器中蛋白质质量控制机制的主要组成部分。与 LonA 肽酶 N 端结构域同源但缺乏其他结构域的蛋白质在包括黄单胞菌目在内的多个原核生物门中是保守的。然而,这些同源蛋白(LonNTD 样蛋白)的功能尚不清楚。此处,以 2.8 Å 分辨率报道了来自野油菜黄单胞菌 (XCC3289;UniProt Q8P5P7)的 LonNTD 样蛋白的晶体结构。该结构通过分子置换解析,在不对称单元中含有一个多肽。结构被精炼至R含量为 29%。XCC3289 的结构由两个通过长环连接的结构域组成。N 端结构域(残基 1-112)由 β 片层包围的 α 螺旋组成,而 C 端结构域(残基 123-193)是 α 螺旋束。这两个结构域的折叠和空间方向与大肠杆菌和鸟分枝杆菌的 LonA 肽酶的 N 末端结构域非常相似。该结构也与 E3 泛素连接酶复合物的底物识别成分 cereblon 相似。已知 LonA 和 cereblon 的 N 末端结构域参与特定的蛋白质-蛋白质相互作用。这种结构分析表明 XCC3289 和其他 LonNTD 样蛋白也可能具有这种蛋白质-蛋白质相互作用的能力。
更新日期:2020-10-02
中文翻译:

野油菜黄单胞菌 XCC3289 的晶体结构:与 Lon 肽酶 N 端底物结合域的同源性
LonA 肽酶是原核生物和真核生物细胞器中蛋白质质量控制机制的主要组成部分。与 LonA 肽酶 N 端结构域同源但缺乏其他结构域的蛋白质在包括黄单胞菌目在内的多个原核生物门中是保守的。然而,这些同源蛋白(LonNTD 样蛋白)的功能尚不清楚。此处,以 2.8 Å 分辨率报道了来自野油菜黄单胞菌 (XCC3289;UniProt Q8P5P7)的 LonNTD 样蛋白的晶体结构。该结构通过分子置换解析,在不对称单元中含有一个多肽。结构被精炼至R含量为 29%。XCC3289 的结构由两个通过长环连接的结构域组成。N 端结构域(残基 1-112)由 β 片层包围的 α 螺旋组成,而 C 端结构域(残基 123-193)是 α 螺旋束。这两个结构域的折叠和空间方向与大肠杆菌和鸟分枝杆菌的 LonA 肽酶的 N 末端结构域非常相似。该结构也与 E3 泛素连接酶复合物的底物识别成分 cereblon 相似。已知 LonA 和 cereblon 的 N 末端结构域参与特定的蛋白质-蛋白质相互作用。这种结构分析表明 XCC3289 和其他 LonNTD 样蛋白也可能具有这种蛋白质-蛋白质相互作用的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号