当前位置:
X-MOL 学术
›
Clin. Exp. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inactivation of TMEM106A promotes lipopolysaccharide‐induced inflammation via the MAPK and NF‐κB signaling pathways in macrophages
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-10-02 , DOI: 10.1111/cei.13528 X Zhang 1, 2, 3 , T Feng 4 , X Zhou 4 , P M Sullivan 4 , F Hu 4 , Y Lou 5 , J Yu 1, 2 , J Feng 1, 2 , H Liu 6 , Y Chen 1, 2, 7
Clinical & Experimental Immunology ( IF 3.4 ) Pub Date : 2020-10-02 , DOI: 10.1111/cei.13528 X Zhang 1, 2, 3 , T Feng 4 , X Zhou 4 , P M Sullivan 4 , F Hu 4 , Y Lou 5 , J Yu 1, 2 , J Feng 1, 2 , H Liu 6 , Y Chen 1, 2, 7
Affiliation
|
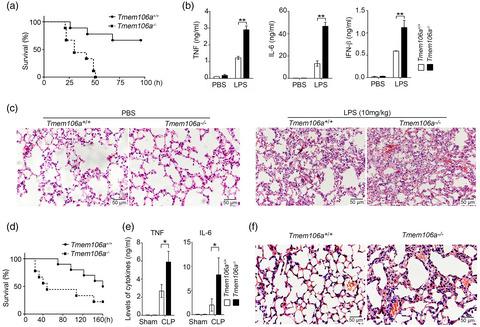
Pattern recognition receptors, such as Toll‐like receptors (TLRs), play an important role in the host defense against invading microbial pathogens. Their activation must be precisely regulated, as inappropriate activation or overactivation of TLR signaling pathways may result in inflammatory disorders, such as septic shock or autoimmune diseases. TMEM106A is a type II transmembrane protein constitutively expressed in macrophages. Our current study demonstrated that TMEM106A levels were increased in macrophages upon lipopolysaccharide (LPS) stimulation, as well as in the peripheral monocytes of patients with sepsis. Tmem106a knockout mice were more sensitive to lipopolysaccharide (LPS)‐induced septic shock than wild‐type mice. Further experiments indicated that Tmem106a ablation enhanced the expression of CD80, CD86 and major histocompatibility complex (MHC)‐II in mouse macrophages upon LPS stimulation, accompanied with up‐regulation of tumor necrosis factor (TNF)‐α, interleukin (IL)‐6, interferon (IFN)‐β and inducible nitric oxide synthase (iNOS), indicating the activation of macrophages and polarization towards the M1 inflammatory phenotype. Moreover, elevated mitogen‐activated protein kinase (MAPK) and nuclear factor kappa B (NF‐κB) signaling were found to be involved in the LPS‐induced inflammatory response in Tmem106a−/− macrophages. However, this effect was largely abrogated by macrophage deletion in Tmem106a−/− mice. Therefore, deficiency of Tmem106a in macrophages may enhance the M1 polarization in mice, resulting in inflammation. This suggests that TMEM106A plays an important regulatory role in maintaining macrophage homeostasis.
中文翻译:

TMEM106A 的失活通过巨噬细胞中的 MAPK 和 NF-κB 信号通路促进脂多糖诱导的炎症
模式识别受体,如 Toll 样受体 (TLR),在宿主防御微生物病原体入侵中发挥重要作用。它们的激活必须受到精确调节,因为 TLR 信号通路的不当激活或过度激活可能导致炎症性疾病,例如败血性休克或自身免疫性疾病。TMEM106A 是一种在巨噬细胞中组成型表达的 II 型跨膜蛋白。我们目前的研究表明,脂多糖 (LPS) 刺激后巨噬细胞中以及脓毒症患者的外周单核细胞中的 TMEM106A 水平增加。与野生型小鼠相比,Tmem106a基因敲除小鼠对脂多糖 (LPS) 诱导的感染性休克更敏感。进一步的实验表明Tmem106a消融增强了 LPS 刺激下小鼠巨噬细胞中 CD80、CD86 和主要组织相容性复合体 (MHC)-II 的表达,同时上调肿瘤坏死因子 (TNF)-α、白细胞介素 (IL)-6、干扰素 (IFN) ‐β 和诱导型一氧化氮合酶 (iNOS),表明巨噬细胞的激活和向 M1 炎症表型的极化。此外,发现升高的丝裂原活化蛋白激酶 (MAPK) 和核因子 kappa B (NF-κB) 信号与Tmem106a -/-巨噬细胞中LPS 诱导的炎症反应有关。然而,Tmem106a -/-小鼠中的巨噬细胞缺失在很大程度上消除了这种影响。因此,Tmem106a 的缺乏在巨噬细胞中可能会增强小鼠的 M1 极化,导致炎症。这表明 TMEM106A 在维持巨噬细胞稳态方面起着重要的调节作用。
更新日期:2020-10-02
中文翻译:

TMEM106A 的失活通过巨噬细胞中的 MAPK 和 NF-κB 信号通路促进脂多糖诱导的炎症
模式识别受体,如 Toll 样受体 (TLR),在宿主防御微生物病原体入侵中发挥重要作用。它们的激活必须受到精确调节,因为 TLR 信号通路的不当激活或过度激活可能导致炎症性疾病,例如败血性休克或自身免疫性疾病。TMEM106A 是一种在巨噬细胞中组成型表达的 II 型跨膜蛋白。我们目前的研究表明,脂多糖 (LPS) 刺激后巨噬细胞中以及脓毒症患者的外周单核细胞中的 TMEM106A 水平增加。与野生型小鼠相比,Tmem106a基因敲除小鼠对脂多糖 (LPS) 诱导的感染性休克更敏感。进一步的实验表明Tmem106a消融增强了 LPS 刺激下小鼠巨噬细胞中 CD80、CD86 和主要组织相容性复合体 (MHC)-II 的表达,同时上调肿瘤坏死因子 (TNF)-α、白细胞介素 (IL)-6、干扰素 (IFN) ‐β 和诱导型一氧化氮合酶 (iNOS),表明巨噬细胞的激活和向 M1 炎症表型的极化。此外,发现升高的丝裂原活化蛋白激酶 (MAPK) 和核因子 kappa B (NF-κB) 信号与Tmem106a -/-巨噬细胞中LPS 诱导的炎症反应有关。然而,Tmem106a -/-小鼠中的巨噬细胞缺失在很大程度上消除了这种影响。因此,Tmem106a 的缺乏在巨噬细胞中可能会增强小鼠的 M1 极化,导致炎症。这表明 TMEM106A 在维持巨噬细胞稳态方面起着重要的调节作用。









































 京公网安备 11010802027423号
京公网安备 11010802027423号