Journal of Organometallic Chemistry ( IF 2.1 ) Pub Date : 2020-10-02 , DOI: 10.1016/j.jorganchem.2020.121544 Wei-Ming Liu , Min Zhang , Jian-Feng Yan , Cai-Xia Lin , Yao-Feng Yuan
|
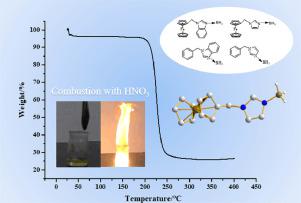
N-(arylmethylene)-benzimidazole/imidazole-borane compounds (7-10) based on ferrocene/benzene were synthesized in a cost-effective way by reacting N-(arylmethylene)-benzimidazole/imidazoles with ammonium sulfate ((NH4)2SO4) and sodium borohydride (NaBH4). The structures of compounds 7-9 were determined by X-ray crystallography. Four compounds displayed high thermal decomposition temperatures (182-256°C) according to the thermogravimetry (TG) measurements. Interestingly, these compounds showed promising applications as potential propellant due to their spontaneous combustion upon contact concentrated HNO3 (≥ 95 %) and catalytic effect on the thermal degradation of ammonium perchlorate (AP).
中文翻译:

N-(芳基亚甲基)-苯并咪唑/咪唑-硼烷化合物的合成与表征
通过使N-(芳基亚甲基)-苯并咪唑/咪唑与硫酸铵((NH 4)2)反应以具有成本效益的方式合成基于二茂铁/苯的N-(芳亚甲基)-苯并咪唑/咪唑-硼烷化合物(7-10) SO 4)和硼氢化钠(NaBH 4)。化合物7-9的结构通过X射线晶体学测定。根据热重(TG)测量,四种化合物显示出高的热分解温度(182-256°C)。有趣的是,这些化合物由于在接触浓HNO 3时自燃,因此显示出潜在的推进剂应用潜力。 (≥95%)和对高氯酸铵(AP)热降解的催化作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号