Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-10-02 , DOI: 10.1016/j.molliq.2020.114442 Maryam Chafiq , Abdelkarim Chaouiki , Mustafa R. Albayati , Hassane Lgaz , Rachid Salghi , Siham K. AbdelRaheem , Ismat H. Ali , Shaaban K. Mohamed , Ill-Min Chung
|
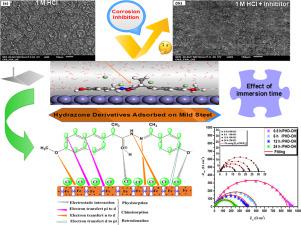
The current investigation seeks to explore the adsorption mechanism of newly synthesized Naproxen-based hydrazones on mild steel (MS) surface in 1.0 M HCl solution and their corrosion inhibition efficiencies. To this end, two hydrazone derivatives namely, (E)-N′-(1-(4-chlorophenyl)ethylidene)-2-(6-methoxynaphthalen-2-yl)propanehydrazide (PHD Cl) and (E)-N′-(1-(4-hydroxyphenyl)ethylidene)-2-(6-methoxynaphthalen-2-yl)propanehydrazide (PHD-OH) were synthesized, characterized and their corrosion inhibition effects were evaluated using a combined electrochemical and theoretical approach. It is evidently clear from the findings presented in this investigation that the two inhibitors exhibited excellent protection efficiency, and the best inhibition performance was shown by PHD-OH inhibitor (96% at 5 × 10−3 M). Weight loss measurements revealed that the optimum concentration of inhibitors is 5 × 10−3 mol/L. The experimental results obtained by electrochemical techniques (potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS)) indicated that the presence of PHD-Cl and PHD-OH compounds greatly increased the polarization resistance and affected both anodic and cathodic reactions, i.e. mixed-type inhibitors. Based on electrochemical results, the polarization resistance was greatly increased, from an initial value for the MS (in 1.0 mol/L HCl) of 29 up to 871 Ω cm2 for the inhibited solution (1.0 mol/L HCl with 5 × 10−3 mol/L of PHD-OH). Furthermore, the adsorption isotherm coincides well with the Langmuir isotherm model. The effect of temperature on PHD-OH adsorption was investigated, experimentally using weight loss tests, and theoretically using molecular dynamic simulations (MD). Moreover, the study found that a protective barrier was set up through the adsorption of the studied compounds on MS surface which is confirmed by scanning electron microscopy with energy-dispersive X-ray analysis (SEM-EDX).Moreover, molecular proprieties of corrosion inhibitor molecules were explored from a theoretical viewpoint using Density Functional Theory (DFT), molecular dynamic (MD) simulation and radial distribution function (RDF) studies. Theoretical results that were in good agreement with experimental findings demonstrated strong interactions between inhibitor molecules and metal surface.
Cl) and (E)-N′-(1-(4-hydroxyphenyl)ethylidene)-2-(6-methoxynaphthalen-2-yl)propanehydrazide (PHD-OH) were synthesized, characterized and their corrosion inhibition effects were evaluated using a combined electrochemical and theoretical approach. It is evidently clear from the findings presented in this investigation that the two inhibitors exhibited excellent protection efficiency, and the best inhibition performance was shown by PHD-OH inhibitor (96% at 5 × 10−3 M). Weight loss measurements revealed that the optimum concentration of inhibitors is 5 × 10−3 mol/L. The experimental results obtained by electrochemical techniques (potentiodynamic polarization (PDP) and electrochemical impedance spectroscopy (EIS)) indicated that the presence of PHD-Cl and PHD-OH compounds greatly increased the polarization resistance and affected both anodic and cathodic reactions, i.e. mixed-type inhibitors. Based on electrochemical results, the polarization resistance was greatly increased, from an initial value for the MS (in 1.0 mol/L HCl) of 29 up to 871 Ω cm2 for the inhibited solution (1.0 mol/L HCl with 5 × 10−3 mol/L of PHD-OH). Furthermore, the adsorption isotherm coincides well with the Langmuir isotherm model. The effect of temperature on PHD-OH adsorption was investigated, experimentally using weight loss tests, and theoretically using molecular dynamic simulations (MD). Moreover, the study found that a protective barrier was set up through the adsorption of the studied compounds on MS surface which is confirmed by scanning electron microscopy with energy-dispersive X-ray analysis (SEM-EDX).Moreover, molecular proprieties of corrosion inhibitor molecules were explored from a theoretical viewpoint using Density Functional Theory (DFT), molecular dynamic (MD) simulation and radial distribution function (RDF) studies. Theoretical results that were in good agreement with experimental findings demonstrated strong interactions between inhibitor molecules and metal surface.
中文翻译:

对盐酸萘普生衍生物在盐酸中对低碳钢缓蚀机理的透彻理解:联合实验/理论研究
当前的研究试图探索新合成的萘普生基在1.0 M HCl溶液中在低碳钢(MS)表面上的吸附机理及其缓蚀效率。为此,两个衍生物,即(E)-N'-(1-(4-氯苯基)亚乙基)-2-(6-甲氧基萘-2-基)丙酰肼(PHD  Cl)和(E)-N'合成了-(1-(4-羟基苯基)亚乙基)-2-(6-甲氧基萘-2-基)丙酰肼(PHD-OH),并结合电化学和理论方法对其腐蚀抑制效果进行了评价。从本研究中发现的结果显然可以看出,这两种抑制剂均表现出优异的保护效率,而PHD-OH抑制剂表现出最佳的抑制性能(5×10时为96%)。-3 M)。失重测量表明抑制剂的最佳浓度为5×10 -3 mol / L。通过电化学技术(电位动力学极化(PDP)和电化学阻抗谱(EIS))获得的实验结果表明,PHD-Cl和PHD-OH化合物的存在大大提高了极化电阻,并影响了阳极和阴极反应,即混合类型抑制剂。基于电化学的结果,极化电阻大大增加,从针对所述MS的初始值(在1.0mol / L的盐酸盐)的29到871Ω厘米2的抑制溶液(1.0 mol / L盐酸,用5×10 - 3 摩尔/升的PHD-OH)。此外,吸附等温线与Langmuir等温线模型非常吻合。研究了温度对PHD-OH吸附的影响,使用失重测试进行了实验,并在理论上使用了分子动力学模拟(MD)。此外,研究发现通过将被研究化合物吸附在MS表面上可以建立保护层,这通过扫描电子显微镜和能量色散X射线分析(SEM-EDX)得以证实。此外,缓蚀剂的分子特性使用密度泛函理论(DFT),分子动力学(MD)模拟和径向分布函数(RDF)研究从理论角度探索了分子。
Cl)和(E)-N'合成了-(1-(4-羟基苯基)亚乙基)-2-(6-甲氧基萘-2-基)丙酰肼(PHD-OH),并结合电化学和理论方法对其腐蚀抑制效果进行了评价。从本研究中发现的结果显然可以看出,这两种抑制剂均表现出优异的保护效率,而PHD-OH抑制剂表现出最佳的抑制性能(5×10时为96%)。-3 M)。失重测量表明抑制剂的最佳浓度为5×10 -3 mol / L。通过电化学技术(电位动力学极化(PDP)和电化学阻抗谱(EIS))获得的实验结果表明,PHD-Cl和PHD-OH化合物的存在大大提高了极化电阻,并影响了阳极和阴极反应,即混合类型抑制剂。基于电化学的结果,极化电阻大大增加,从针对所述MS的初始值(在1.0mol / L的盐酸盐)的29到871Ω厘米2的抑制溶液(1.0 mol / L盐酸,用5×10 - 3 摩尔/升的PHD-OH)。此外,吸附等温线与Langmuir等温线模型非常吻合。研究了温度对PHD-OH吸附的影响,使用失重测试进行了实验,并在理论上使用了分子动力学模拟(MD)。此外,研究发现通过将被研究化合物吸附在MS表面上可以建立保护层,这通过扫描电子显微镜和能量色散X射线分析(SEM-EDX)得以证实。此外,缓蚀剂的分子特性使用密度泛函理论(DFT),分子动力学(MD)模拟和径向分布函数(RDF)研究从理论角度探索了分子。











































 京公网安备 11010802027423号
京公网安备 11010802027423号