当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Total syntheses of spiroviolene and spirograterpene A: a structural reassignment with biosynthetic implications
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-30 , DOI: 10.1039/d0sc04686h Hyung Min Chi 1 , Charles J F Cole 1 , Pengfei Hu 1 , Cooper A Taylor 1 , Scott A Snyder 1
Chemical Science ( IF 7.6 ) Pub Date : 2020-09-30 , DOI: 10.1039/d0sc04686h Hyung Min Chi 1 , Charles J F Cole 1 , Pengfei Hu 1 , Cooper A Taylor 1 , Scott A Snyder 1
Affiliation
|
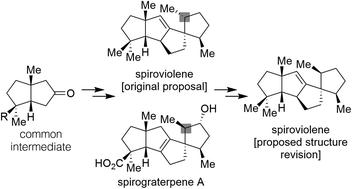
The recent natural product isolates spiroviolene and spirograterpene A are two relatively non-functionalized linear triquinane terpenes with a large number of structural homologies. Nevertheless, three significant areas of structural disparity exist based on their original assignments, one of which implies a key stereochemical divergence early in their respective biosyntheses. Herein, using two known bicyclic ketone intermediates, a core Pd-catalyzed Heck cyclization sequence, and several chemoselective transformations, we describe concise total syntheses of both natural product targets and propose that the structure of spiroviolene should be reassigned. As a result, these natural products possess greater homology than previously anticipated.
中文翻译:

螺紫烯和螺环烯 A 的全合成:具有生物合成意义的结构重新分配
最近分离出来的天然产物螺紫烯和螺环萜烯A是两种相对非功能化的线性三奎烷萜烯,具有大量的结构同源性。然而,根据它们最初的分配,存在三个显着的结构差异领域,其中之一意味着它们各自的生物合成早期存在关键的立体化学分歧。在此,使用两种已知的双环酮中间体、核心 Pd 催化的 Heck 环化序列和几种化学选择性转化,我们描述了两种天然产物目标的简明全合成,并提出应重新分配螺紫烯的结构。因此,这些天然产物具有比之前预期更高的同源性。
更新日期:2020-10-14
中文翻译:

螺紫烯和螺环烯 A 的全合成:具有生物合成意义的结构重新分配
最近分离出来的天然产物螺紫烯和螺环萜烯A是两种相对非功能化的线性三奎烷萜烯,具有大量的结构同源性。然而,根据它们最初的分配,存在三个显着的结构差异领域,其中之一意味着它们各自的生物合成早期存在关键的立体化学分歧。在此,使用两种已知的双环酮中间体、核心 Pd 催化的 Heck 环化序列和几种化学选择性转化,我们描述了两种天然产物目标的简明全合成,并提出应重新分配螺紫烯的结构。因此,这些天然产物具有比之前预期更高的同源性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号