当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dolabellane and Clerodane Diterpenoids from the Twigs and Leaves of Casearia kurzii
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-10-01 , DOI: 10.1021/acs.jnatprod.9b00427 Long-Teng Zhang 1 , Xiao-Ling Wang 2 , Tian Wang 1 , Jun-Sheng Zhang 3 , Zhu-Qing Huang 1 , Tao Shen 1 , Hong-Xiang Lou 1 , Dong-Mei Ren 1 , Xiao-Ning Wang 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-10-01 , DOI: 10.1021/acs.jnatprod.9b00427 Long-Teng Zhang 1 , Xiao-Ling Wang 2 , Tian Wang 1 , Jun-Sheng Zhang 3 , Zhu-Qing Huang 1 , Tao Shen 1 , Hong-Xiang Lou 1 , Dong-Mei Ren 1 , Xiao-Ning Wang 1
Affiliation
|
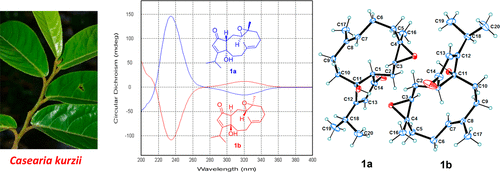
A pair of enantiomeric 15-nordolabellane diterpenoids, (−)- and (+)-caseadolabellols A (1a and 1b), three dolabellane diterpenoids, caseadolabellols B–D (2–4), two dolastane diterpenoids, caseadolastols A and B (5 and 6), 10 clerodane diterpenoids, caseakurzins A–J (7–16), and nine known diterpenoids (17–25) were isolated from the twigs and leaves of Casearia kurzii. The structures of the new compounds were established on the basis of extensive spectroscopic data, and those of compounds 1a, 1b, and 2 were verified by single-crystal X-ray crystallographic analysis. The enantiomers 1a and 1b were separated by chiral-phase HPLC. The absolute configurations were determined by experimental and calculated ECD data, the modified Mosher’s method, or literature comparison. Compounds 1a and 5 showed significant quinone reductase-inducing activity in Hepa 1c1c7 cells, while 1b showed moderate activity. Molecular docking studies showed that 1a had greater binding affinity with Nrf2 protein (5FNQ) than 1b. The cytotoxic activity of compounds 1a, 1b, 2–12, 15, and 16 was evaluated, among which compounds 8 and 16 exhibited significant inhibitory activity against the A549 cell line. Compounds 8 and 16 induced the A549 cells to arrest at G2/M and S phases, respectively, and both compounds induced apoptosis in A549 cells.
中文翻译:

来自 Casearia kurzii 的树枝和叶子的 Dolabellane 和 Clerodane 二萜
一对对映异构体 15-nordolabellane 二萜、(-)- 和 (+)-caseadolabellols A(1a和1b)、三种 dolabellane 二萜、caseadolabellols B–D(2 – 4)、两种 dolastane 二萜、caseadolastols A 和 B(5和6),10个克罗二萜类化合物,caseakurzins A〜J(7 - 16),和九个已知二萜类化合物(17 - 25)从的枝叶分离嘉赐kurzii。新化合物的结构是在大量光谱数据的基础上建立的,化合物1a、1b和2经单晶 X 射线晶体学分析验证。对映异构体1a和1b通过手性相 HPLC 进行分离。绝对配置由实验和计算 ECD 数据、修改后的 Mosher 方法或文献比较确定。化合物1a和5在 Hepa 1c1c7 细胞中显示出显着的醌还原酶诱导活性,而1b显示出中等活性。分子对接研究表明,1a与 Nrf2 蛋白 (5FNQ) 的结合亲和力高于1b。的化合物的细胞毒性活性1A,1B,2 - 12,15,和16进行评价,其中化合物8和16针对A549细胞系表现出显著抑制活性。化合物8和16分别诱导A549细胞在G2/M和S期停滞,并且两种化合物均诱导A549细胞凋亡。
更新日期:2020-10-26
中文翻译:

来自 Casearia kurzii 的树枝和叶子的 Dolabellane 和 Clerodane 二萜
一对对映异构体 15-nordolabellane 二萜、(-)- 和 (+)-caseadolabellols A(1a和1b)、三种 dolabellane 二萜、caseadolabellols B–D(2 – 4)、两种 dolastane 二萜、caseadolastols A 和 B(5和6),10个克罗二萜类化合物,caseakurzins A〜J(7 - 16),和九个已知二萜类化合物(17 - 25)从的枝叶分离嘉赐kurzii。新化合物的结构是在大量光谱数据的基础上建立的,化合物1a、1b和2经单晶 X 射线晶体学分析验证。对映异构体1a和1b通过手性相 HPLC 进行分离。绝对配置由实验和计算 ECD 数据、修改后的 Mosher 方法或文献比较确定。化合物1a和5在 Hepa 1c1c7 细胞中显示出显着的醌还原酶诱导活性,而1b显示出中等活性。分子对接研究表明,1a与 Nrf2 蛋白 (5FNQ) 的结合亲和力高于1b。的化合物的细胞毒性活性1A,1B,2 - 12,15,和16进行评价,其中化合物8和16针对A549细胞系表现出显著抑制活性。化合物8和16分别诱导A549细胞在G2/M和S期停滞,并且两种化合物均诱导A549细胞凋亡。











































 京公网安备 11010802027423号
京公网安备 11010802027423号