当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Beyond Transition Metal Oxide Cathodes for Electric Aviation: The Case of Rechargeable CFx
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-10-01 , DOI: 10.1021/acsenergylett.0c01815 Venkatesh Krishnamurthy 1 , Venkatasubramanian Viswanathan 1, 2
ACS Energy Letters ( IF 19.3 ) Pub Date : 2020-10-01 , DOI: 10.1021/acsenergylett.0c01815 Venkatesh Krishnamurthy 1 , Venkatasubramanian Viswanathan 1, 2
Affiliation
|
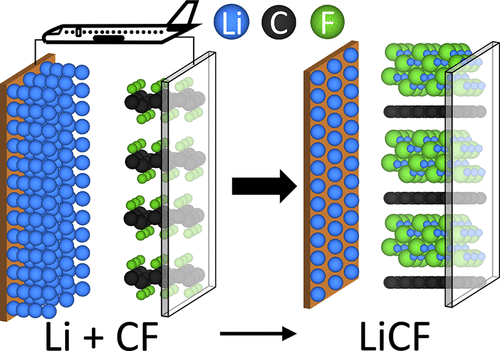
Current and next-generation transition metal oxide-based rechargeable battery chemistries are likely to fall short of the specific energy needed to electrify aircraft. One approach to enabling electric aviation is making high specific energy primary battery chemistries such as the Li-CFx chemistry rechargeable. Though Li-CFx possesses nearly triple the specific energy of current Li-ion cells, numerous fundamental issues related to the overall reaction mechanism exist. In this work, we use density functional theory calculations to build a fundamental understanding of possible reaction mechanisms. The direct formation of LiF and graphite seems unlikely because of the sluggish kinetics of F diffusion in CFx. The discharge occurs likely via lithium ion diffusion into the CFx host to form an LiCF ternary compound. Reasonable agreement between the open-circuit voltage (OCV) determined experimentally (∼4.05 V) and from DFT (4.27 ± 0.14 V) for the formation of ternary LiCF is obtained. Suppressing LiF formation by stabilizing the intermediate ternary LiCF could thus enable rechargeability of the Li-CFx chemistry.
中文翻译:

超越用于航空电子的过渡金属氧化物阴极:可充电CF x的情况
当前和下一代基于过渡金属氧化物的可充电电池化学物质可能无法满足飞机电气化所需的比能。实现电动航空的一种方法是使高比能一次电池化学物质如Li-CF x化学物质可充电。尽管Li-CF x的比能量几乎是当前锂离子电池的三倍,但仍存在许多与总体反应机理有关的基本问题。在这项工作中,我们使用密度泛函理论计算来建立对可能的反应机理的基本理解。LiF和石墨的直接形成似乎不太可能,因为CF x中F扩散的动力学缓慢。可能通过锂离子扩散到CF x主体中形成LiCF三元化合物而发生放电。获得了实验确定的开路电压(OCV)(〜4.05 V)和DFT(4.27±0.14 V)形成三元LiCF的合理协议。因此,通过稳定中间三元LiCF抑制LiF的形成可以使Li-CF x化学物的可再充电性成为可能。
更新日期:2020-11-13
中文翻译:

超越用于航空电子的过渡金属氧化物阴极:可充电CF x的情况
当前和下一代基于过渡金属氧化物的可充电电池化学物质可能无法满足飞机电气化所需的比能。实现电动航空的一种方法是使高比能一次电池化学物质如Li-CF x化学物质可充电。尽管Li-CF x的比能量几乎是当前锂离子电池的三倍,但仍存在许多与总体反应机理有关的基本问题。在这项工作中,我们使用密度泛函理论计算来建立对可能的反应机理的基本理解。LiF和石墨的直接形成似乎不太可能,因为CF x中F扩散的动力学缓慢。可能通过锂离子扩散到CF x主体中形成LiCF三元化合物而发生放电。获得了实验确定的开路电压(OCV)(〜4.05 V)和DFT(4.27±0.14 V)形成三元LiCF的合理协议。因此,通过稳定中间三元LiCF抑制LiF的形成可以使Li-CF x化学物的可再充电性成为可能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号