当前位置:
X-MOL 学术
›
Polym. Int.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
New sustainable alternating semi‐aromatic polyamides prepared in bulk by direct solid‐state polymerization
Polymer International ( IF 2.9 ) Pub Date : 2020-10-01 , DOI: 10.1002/pi.6134 Martin Wolffs 1 , Lucy Cotton 1 , Ard J. Kolkman 1 , Rudy Rulkens 1
Polymer International ( IF 2.9 ) Pub Date : 2020-10-01 , DOI: 10.1002/pi.6134 Martin Wolffs 1 , Lucy Cotton 1 , Ard J. Kolkman 1 , Rudy Rulkens 1
Affiliation
|
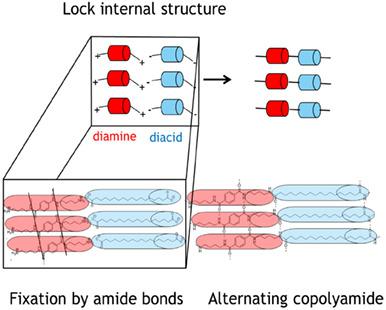
New semi‐aromatic bio‐based copolyamides PA 4T/4Y and PA 6T/6Y were successfully synthesized in bulk by direct solid‐state polycondensation in which hydrophobic bio‐based dicarboxylic acids sebacic acid, octadecanedioic or hydrogenated dimer fatty acid (Y = C10, C18 or C36) alternate with terephthalic acid T. The absence of a polymerization solvent and the possibility of performing the polymerization on the dicarboxylic acid make it an interesting route for future production of alternating copolyamides. A stepwise synthetic approach is taken where first terephthalic acid‐based diamide diamines of the linear C4 or C6 diamines 4T4 or 6T6 are prepared, followed by preparation of solid salts with the above aliphatic dicarboxylic acids and subjecting the salts to a solid‐state post‐condensation. The alternation is achieved by the interlocking of the 4T4 or 6T6 units in the salts with the bio‐based dicarboxylic acids and polymerizing these salts below the melt temperature of the terephthalamide‐based core. During the polycondensation, the transamidation reaction of the terephthalamide moiety is prevented and with that the randomization of the polyamide. Especially the diaminobutane‐based PA 4T/4Y copolyamides show strict alternation, leading to interesting properties like high crystallinity and high melting points of 250 to 318 °C supported by a randomness value of 2, determined using 13C NMR spectroscopy. The special alternating feature becomes especially clear when heating the polymer in the melt state, when the transamidation of the terephthalamide moieties is no longer prevented by the interlocking principle, leading to lowering of the randomness value and therewith the melting point and crystallinity. © 2020 Society of Industrial Chemistry
中文翻译:

通过直接固态聚合本体制备的新型可持续交替半芳族聚酰胺
新型半芳族生物基共聚酰胺PA 4T / 4Y和PA 6T / 6Y通过直接固态缩聚反应成功地批量合成,其中疏水性生物基二羧酸癸二酸,十八烷二酸或氢化二聚脂肪酸(Y = C10, C18或C36)与对苯二甲酸T交替存在。不存在聚合溶剂和对二羧酸进行聚合的可能性使其成为将来生产交替共聚酰胺的有趣途径。采用逐步合成的方法,首先制备线性C4或C6二胺4T4或6T6的对苯二甲酸二酰胺二胺,然后与上述脂肪族二羧酸制备固体盐,然后将盐进行固态后处理。缩合。通过将盐中的4T4或6T6单元与生物基二元羧酸互锁,并使这些盐在对苯二甲酰胺基核的熔融温度以下进行聚合,可以实现这种交替。在缩聚过程中,防止了对苯二甲酰胺部分的氨基转移反应,从而防止了聚酰胺的无规化。尤其是基于二氨基丁烷的PA 4T / 4Y共聚酰胺表现出严格的交替性,从而产生了有趣的特性,例如高结晶度和250至318°C的高熔点,并由2的随机性值支持。防止了对苯二甲酰胺部分的氨基转移反应,从而防止了聚酰胺的无规化。尤其是基于二氨基丁烷的PA 4T / 4Y共聚酰胺表现出严格的交替性,从而产生了有趣的特性,例如高结晶度和250至318°C的高熔点,并由2的随机性值支持。防止了对苯二甲酰胺部分的氨基转移反应,从而防止了聚酰胺的无规化。尤其是基于二氨基丁烷的PA 4T / 4Y共聚酰胺表现出严格的交替性,从而产生了有趣的特性,例如高结晶度和250至318°C的高熔点,并由2的随机性值支持。 13 C NMR光谱。当以熔融状态加热聚合物时,当互锁原理不再阻止对苯二甲酰胺基团的氨基转移时,该特殊的交替特征变得特别明显,从而导致无规度值以及随之降低的熔点和结晶度。©2020工业化学学会
更新日期:2020-10-01
中文翻译:

通过直接固态聚合本体制备的新型可持续交替半芳族聚酰胺
新型半芳族生物基共聚酰胺PA 4T / 4Y和PA 6T / 6Y通过直接固态缩聚反应成功地批量合成,其中疏水性生物基二羧酸癸二酸,十八烷二酸或氢化二聚脂肪酸(Y = C10, C18或C36)与对苯二甲酸T交替存在。不存在聚合溶剂和对二羧酸进行聚合的可能性使其成为将来生产交替共聚酰胺的有趣途径。采用逐步合成的方法,首先制备线性C4或C6二胺4T4或6T6的对苯二甲酸二酰胺二胺,然后与上述脂肪族二羧酸制备固体盐,然后将盐进行固态后处理。缩合。通过将盐中的4T4或6T6单元与生物基二元羧酸互锁,并使这些盐在对苯二甲酰胺基核的熔融温度以下进行聚合,可以实现这种交替。在缩聚过程中,防止了对苯二甲酰胺部分的氨基转移反应,从而防止了聚酰胺的无规化。尤其是基于二氨基丁烷的PA 4T / 4Y共聚酰胺表现出严格的交替性,从而产生了有趣的特性,例如高结晶度和250至318°C的高熔点,并由2的随机性值支持。防止了对苯二甲酰胺部分的氨基转移反应,从而防止了聚酰胺的无规化。尤其是基于二氨基丁烷的PA 4T / 4Y共聚酰胺表现出严格的交替性,从而产生了有趣的特性,例如高结晶度和250至318°C的高熔点,并由2的随机性值支持。防止了对苯二甲酰胺部分的氨基转移反应,从而防止了聚酰胺的无规化。尤其是基于二氨基丁烷的PA 4T / 4Y共聚酰胺表现出严格的交替性,从而产生了有趣的特性,例如高结晶度和250至318°C的高熔点,并由2的随机性值支持。 13 C NMR光谱。当以熔融状态加热聚合物时,当互锁原理不再阻止对苯二甲酰胺基团的氨基转移时,该特殊的交替特征变得特别明显,从而导致无规度值以及随之降低的熔点和结晶度。©2020工业化学学会











































 京公网安备 11010802027423号
京公网安备 11010802027423号